
Learn More About the Super Rare Disease – Esophageal Diverticulum
Why am I Writing About the Rare Disease, Esophageal Diverticulum? The short answer is because I was recently diagnosed with it, although symptoms have been accumulating for some time now. I had simply assumed that all the regurgitation and vomiting, even in the middle of […]
Chronic voicesGluten-Free Christmas Dinner Recipes for a Delicious Holiday Feast
The Christmas season has arrived and so have these delicious gluten-free Christmas Dinner recipes. In this recipe roundup, you’ll find a collection of savory gluten-free Christmas main dishes to delight your guests and make your holiday meal unforgettable. Pair them with the delicious assortment of […]
mind-&-emotions
MediSearch Review: A Medical AI Search Engine for Patients, Clinicians & Medical Writers
Introduction to MediSearch, a Medical AI Search Engine You’ve probably asked ChatGPT, Gemini or another AI-powered chatbot a medical or health-related question before. Perhaps to try and find a diagnosis, or simply out of curiosity. I have personally asked them some medical questions that I […]
Chronic voices
20 Best Pumpkin Dessert Recipes to Indulge in This Fall
20 Pumpkin Dessert Recipes to Indulge in This Fall Pumpkin season is upon us and so are these delicious pumpkin dessert recipes. Feeling pessimistic because of your paleo diet? Gloomy because you’re gluten-free? Well fret not. This recipe roundup includes an assortment of gluten-free and […]
mind-&-emotions20 Pumpkin Dessert Recipes to Indulge in This Fall
Pumpkin season is upon us and so are these delicious pumpkin dessert recipes. Feeling pessimistic because of your paleo diet? Gloomy because you’re gluten-free? Well fret not. This recipe roundup includes an assortment of gluten-free and paleo pumpkin desserts you’re sure to love.

Why Pumpkin Fall Recipes Are a Must for Healthy Autumn Indulgence
Why do we love fall pumpkin recipes so much? Is it because the smell of pumpkin and spices makes us feel warm and cozy?
Maybe pumpkins remind us of the upcoming holiday season.
Well, here’s another reason to love pumpkins. Pumpkins are loaded with antioxidants and a wide variety of vitamins and nutrients. Vitamins A, C , E, B6, iron, magnesium, niacin and folate are just a few.
Pumpkins are also a rich source of fiber which helps improve gut health. And since pumpkins are naturally sweet and low in calories, they’re a good choice if you’re trying to maintain your weight.
Pumpkin And Warm Spices: The Perfect Pair for Gluten-Free and Paleo Baking
Pumpkins pair nicely with warm spices like nutmeg, cinnamon, and cloves. The comforting flavor and aroma are so inviting, even candle companies have capitalized on the scent.
If you’re on the gluten-free or paleo diet, you’re in for a special treat. Not only do pumpkins help baked recipes retain moisture. Pumpkins act as a binder, holding your ingredients together. This is important because baked desserts can be crumbly without gluten.
Plus, pumpkins are naturally creamy which makes them the perfect ingredient for recipes without butter or milk.
So brace yourself…even reading these pumpkin dessert recipes can trigger an irresistible craving for autumnal sweetness.
20 Pumpkin Dessert Recipes for Fall




















You May Also Want To Read
Quick and Easy Ninja Foodi Collard Greens With Bacon Recipe
30+ Amazing Gluten-Free Soup Recipes to Warm Up Your Fall and Winter
Summary
There you have it…20 pumpkin dessert recipes to indulge in this fall. This just goes to show, you can have your cake and eat it too on the paleo and gluten-free diets. So grab your apron, pick your favorite recipes and experiment to your heart’s content. Happy baking!
The post 20 Best Pumpkin Dessert Recipes to Indulge in This Fall appeared first on Health Uprising Now.
Paleo vs. Whole30 Diet: Everything You Need to Know
A number of diets have gained popularity in recent years for their ability to reduce inflammation. Inflammation has been associated with autoimmune disease and other chronic illnesses. Two popular diets are the paleo diet and the Whole30 diet. This article will compare the paleo vs. […]
mind-&-emotionsA number of diets have gained popularity in recent years for their ability to reduce inflammation. Inflammation has been associated with autoimmune disease and other chronic illnesses. Two popular diets are the paleo diet and the Whole30 diet. This article will compare the paleo vs. Whole30 diet and explore the pros, cons and purposes of each.
The information provided is for general informational and educational purposes only. It is not intended as medical advice, and it should not be used to diagnose or treat any health condition or illness. Always seek the advice of a qualified healthcare provider with any questions you may have regarding a medical condition or your individual health.

Paleo vs. Whole30 Diet
Chronic inflammation can damage certain types of cells throughout the body. This can ultimately lead to skin disorders, respiratory issues, type 2 diabetes, heart disease, autoimmune disease, and a host of other illnesses.
Both the paleo and Whole30 diets can result in improved health outcomes. But they both have different approaches. The ideal diet for you will depend on your health goals and personal preference.
Purpose of the paleo diet
The paleo diet consists of foods similar to what our hunter-gatherer ancestors ate during the Paleolithic Era.
The premise is that our bodies have not adapted to our “modern diets”. Farming was introduced around 10,000 years ago. Grains, legumes and dairy products became readily available as a result.
It’s believed that our genes were not wired to process these “new” foods. These foods are believed to cause inflammation. Chronic conditions can result.
The paleo diet helps improve health outcomes by eating foods our bodies are wired to eat.
Purpose of Whole30 diet
The purpose of the Whole30 diet is to identify foods you’re intolerant to. Food intolerance can trigger or exacerbate many chronic conditions.
The paleo diet eliminates all potentially inflammatory foods. Forever.
The Whole30 diet eliminates potentially inflammatory foods for 30 days. Once the 30 days are complete, foods are reintroduced. This phase lasts 10 days.
Only foods that cause a reaction on the Whole30 diet are eliminated for good.
Foods you can eat on the paleo and Whole30 diets

Allowed foods on both diets are similar, however, Whole30 is more restrictive. The following foods can be consumed on each diet.
Lean Meats and Grass-Fed Meats
Meats should preferably be grass-fed, free-range, and organic. These meats provide a higher quality of protein and omega-3 than regular meats. The high omega-3 helps to reduce inflammation. But if grass-fed, free-range and organic aren’t available or within your budget, don’t let that stop you. What matters is the meat is unprocessed.
Wild-Caught Fish
These include fish, shellfish and other types of seafood that were harvested directly from natural bodies of water including seas, oceans, and rivers. Halibut, cod, sockeye salmon, and mackerel, are a few examples of wild-caught fish. Wild-caught is preferred over farm-raised, however let your personal preference guide your selection.
Healthy Fats
Healthy fats come from certain oils as well as avocados, nuts, seeds and some fish. Here are some examples.
- Olive oil, coconut oil and avocado oil are considered healthy fats.
- Salmon, tuna, sardines and mackerel are high in omega-3 fatty acids.
- Flax seeds, chia seeds, pumpkin seeds, and sunflower seeds are also healthy fats.
Vegetables
Most vegetables are acceptable on both the paleo and Whole30 diets, including green leafy vegetables, cruciferous vegetables like cabbage and brussel sprouts, starchy vegetables, and root vegetables. However, certain vegetables should be avoided, and they’re mentioned below.
Fruits
Most fruits are allowed on both diets. This includes berries, pits (peaches, plums), cores (apples, pears), citrus fruits, melons and tropical fruit (mangos, papaya). Some stricter paleo eaters will avoid or limit their consumption of fruits containing high amounts of sugar. These sweeter fruits can cause spikes in blood sugar and increased insulin production which can lead to insulin resistance.
Seeds and Nuts
All tree nuts, nut milk, and nut butters are acceptable on both diets. Peanuts, however, are considered legumes and should be avoided. More on restrictions later.
Other
In addition to the above, the following may be consumed in moderation on the paleo diet:
- Dark chocolate, providing it doesn’t have added sugar or milk.
- Wine
- Natural sugars like raw honey, maple syrup, and blackstrap molasses.
You May Also Want To Read
Free Constipation Printable List of High Fiber Foods
Low FODMAP Diet Provides Proven Results for Multiple Chronic Conditions
Gut Health and the Immune System: What You Need to Know
Foods to avoid on the paleo diet and Whole30 diet

The following foods should be avoided on both diets.
Grains
These foods tend to have a high glycemic index and are difficult for some people to digest.
Legumes
These foods contain anti-nutrients which prevent the absorption of nutrients in other foods.
Dairy
Many people are unable to digest milk. In addition, conventional dairy products often contain hormones and antibiotics.
Refined Sugar
Refined sugar causes spikes in blood sugar and insulin levels. Artificial sugar can throw gut bacteria out of balance. This can result in digestive problems as well as metabolic disturbances like insulin resistance, hyperglycemia, hypoglycemia, and hormonal imbalance.
Processed Foods
These foods lack nutrients and tend to be higher in calories. Some people even experience allergic reactions to the artificial colors, flavors, and preservatives in these foods. Processed foods include lunch meats, frozen food, ready made breakfast cereals, and canned fruits, vegetables and meats. Meat products like sausage, bacon, and salami are also processed.
Vegetable Oils
These oils tend to be higher in Omega-6 fatty acids, which makes them more likely to trigger inflammation. In addition, it’s not uncommon for partially hydrogenated oil to contain trans-fats, which are the worst fats.
Other
In addition to the above, the following foods should be avoided on the Whole30 diet.
- ALL Alcohol
- ALL added sugars, including natural sugars like raw honey, maple syrup, and blackstrap molasses
What are the pros of the paleo diet
The paleo diet is rich in nutrient dense foods that promote healing and overall health. Because processed foods are minimized or eliminated, the risks associated with these foods are also reduced. The paleo diet works well for reducing inflammation.
Blood sugar is stabilized with the elimination of refined sugars and carbohydrates. Stable blood sugar means no spikes and crashes that drain you of energy. Many have experienced improvements in mental clarity as a result.
Another benefit is weight loss, since the paleo diet is so restrictive. And there’s no calorie counting involved. Also, nutrient dense foods cause you to feel fuller faster so they support weight maintenance.
The increased fiber from fruits and vegetables helps improve digestive health.
What are the pros of the Whole30 diet
Like the paleo diet, the Whole30 diet is rich in nutrients and helps stabilize blood sugar. It also results in weight loss and improved digestive health. An added benefit of the Whole30 diet is it helps to identify food intolerances.
The Whole30 diet is also said to reset your taste buds, so sugar and salt cravings diminish.
What are the cons of the paleo diet
The paleo diet is a restrictive diet and may be difficult to sustain for the long term. The elimination of grains, legumes, and dairy can result in deficiencies in calcium, vitamin D, B complex vitamins, magnesium and iron.
Reduced carbohydrates can also lead to a lack of energy.
Another disadvantage is that constipation can occur because of the elimination of grains and legumes.
Health isn’t the only concern. A restrictive diet can impact your social life. It may be difficult finding foods you can enjoy while eating out.
Still another con of the paleo diet is the cost. Organic, grass-fed, and wild caught foods can be pricey. Also, these items may not be available where you shop.
Weight loss may not be a desired outcome for some.
The good news is the paleo diet doesn’t have to be all or nothing. You can customize it to suit your needs and preferences.
Years ago I was searching for an anti-inflammatory diet. I opted out of the paleo diet because it seemed too restrictive. Having multiple food allergies and sensitivities, the last thing I needed was a restrictive diet.
But apparently there are different variations of the paleo diet. Some people use the paleo diet as a model to base your diet off of, rather than a rigid set of rules.
What are the cons of the Whole30 diet
Like the paleo diet, the Whole30 diet is highly restrictive, albeit for 30 days. Still there’s a chance of nutritional imbalance as entire food groups are removed. Low energy, constipation, and cost are also cons.
Another con is the potential for rebound weight-gain when restricted foods are added back in.
Is paleo gluten-free
The paleo diet restricts all grains, so it is gluten free. Care should be taken to check labels on foods and seasonings.i
Is Whole30 gluten-free
The Whole30 diet is gluten-free during the 30 day elimination period. However, it can be added back in during the 10 day reintroduction period to see if it is tolerated.
Summary
In a comparison of the paleo vs. Whole30 diet, both emphasize unprocessed, nutrient dense foods. Both diets allow lean meats, fish, fruits, vegetables, nuts, and seeds. Likewise, both diets exclude grains, dairy and legumes. The Whole30 diet is an elimination diet. It has more restrictions, but only during the first 30 days. The paleo diet is more of a lifestyle diet that lasts indefinitely. Each has the potential for reduced inflammation and improved health outcomes. Consult with your healthcare provider to determine the diet that best suits your needs.
The post Paleo vs. Whole30 Diet: Everything You Need to Know appeared first on Health Uprising Now.
High Fiber Gluten-Free Breakfast Recipes to Start Your Day Right
What better way to kick start your day than to indulge in a nutritious high-fiber breakfast. Not only will your digestive system be happy, but you’ll feel satiated and energized. Finding a variety of gluten free recipes that are also high in fiber can be […]
mind-&-emotionsWhat better way to kick start your day than to indulge in a nutritious high-fiber breakfast. Not only will your digestive system be happy, but you’ll feel satiated and energized. Finding a variety of gluten free recipes that are also high in fiber can be challenging. That’s why we created this flavorful roundup of gluten free high fiber breakfast recipes. We’re sure you’ll agree, somewhere in this list is a new morning favorite or two…maybe even three!

What are the benefits of eating a high fiber breakfast
There are many benefits to eating a high fiber breakfast. For starters, a high fiber breakfast fills you up. This reduces the likelihood of you experiencing mid-morning hunger. And if you’re not hungry, you’ll be less tempted to fill up on unhealthy snacks.
Another benefit of eating a high fiber breakfast is that it helps to moderate glucose levels. This prevents spikes and crashes and maintains consistent energy levels.
If you’re prone to constipation, a high fiber breakfast can kickstart your digestive system. Combined with a few simple constipation relief strategies, you can finally get a handle on chronic constipation once and for all.
A diet high in fiber has other health benefits including reducing cholesterol levels and reducing the risk of type 2 diabetes.
Learn more about the benefits of a high fiber diet and grab your Free Constipation List of High Fiber Foods. This list is also gluten-free.
If you’re looking for gluten-free high-fiber dinner recipes, check out Gluten-Free High Fiber Dinner Recipes.
You May Also Want To Read
Gluten-Free High Fiber Dinner Recipes
Free Constipation Printable List of High Fiber Foods
How to Beat Constipation Naturally: 6 Effective Solutions Without Laxatives
Best Probiotic for Gluten Intolerance: A Comprehensive Guide
Low FODMAP Diet Provides Proven Results for Multiple Chronic Conditions















Summary
We hope you’re inspired by these gluten-free high-fiber breakfast recipes. Whether you have a busy day in the office, or a leisurely day at home, you’re sure to find the right recipe that suits your schedule and your tastebuds. Enjoy experimenting and discover first hand the benefits of eating a fiber-rich breakfast.
The post High Fiber Gluten-Free Breakfast Recipes to Start Your Day Right appeared first on Health Uprising Now.
20 Gluten-Free High Fiber Dinner Recipes for Better Health
End your day on a healthy note with our gluten-free high-fiber dinner recipes! Fiber has many health benefits including constipation relief, weight management and sugar regulation. Fiber also minimizes the risk of colon cancer and cardiovascular disease. Learn more about the benefits of fiber and […]
mind-&-emotionsEnd your day on a healthy note with our gluten-free high-fiber dinner recipes!
Fiber has many health benefits including constipation relief, weight management and sugar regulation. Fiber also minimizes the risk of colon cancer and cardiovascular disease. Learn more about the benefits of fiber and download your free constipation printable list of high fiber foods.
This recipe roundup includes an assortment of gluten-free salads, stews, and even stir fry! Made with an assortment of fresh vegetables, gluten-free grains, and beans, these mouthwatering dinner recipes are sure to delight the taste buds. This is the first part of a two-part series.
Stay tuned for the second part of this series on Gluten Free High Fiber Breakfast Recipes.

Gluten-Free High Fiber Dinner Recipes













You May Also Want To Read
How to Beat Constipation Naturally: 6 Effective Solutions Without Laxatives
Free Constipation Printable List of High Fiber Foods
High Fiber Gluten-Free Breakfast Recipes to Start Your Day Right
Best Probiotic for Gluten Intolerance: A Comprehensive Guide
Gut Health and the Immune System: What You Need to Know







We hope you’ve enjoyed exploring our gluten-free high-fiber dinner recipes! Incorporating more fiber while maintaining a gluten-free diet doesn’t have to be challenging. Wind your down day down with a wholesome, delicious dinner that will keep you satisfied and energized while improving overall health. Happy cooking and here’s to a healthier, more fiber-filled diet!
The post 20 Gluten-Free High Fiber Dinner Recipes for Better Health appeared first on Health Uprising Now.

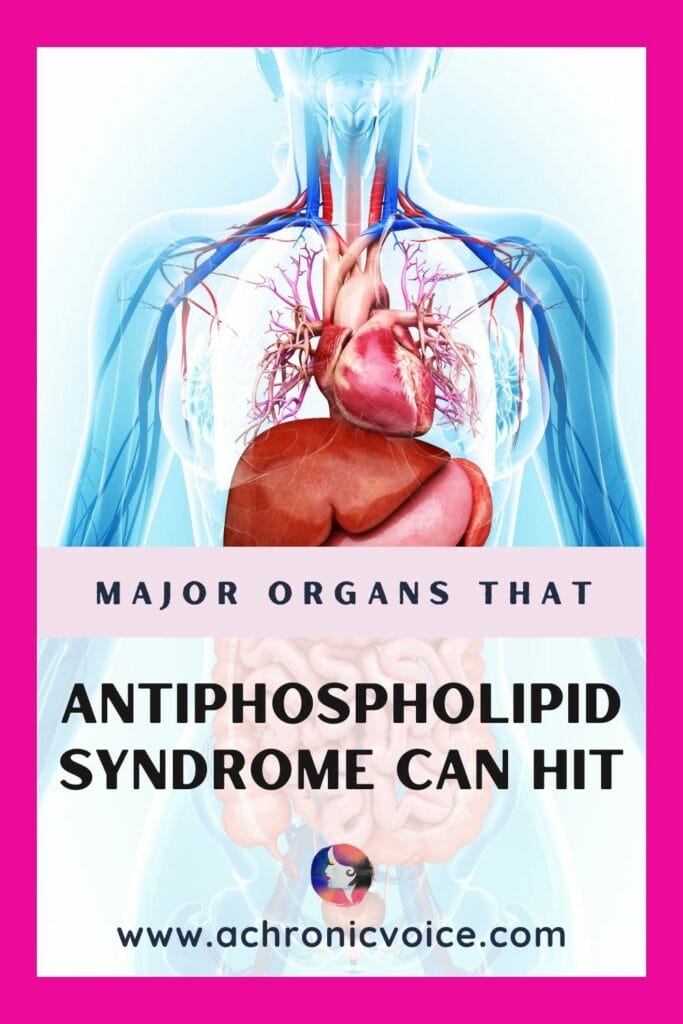
How Does Antiphospholipid Syndrome Affect The Body? (Beyond the Blood to Major Organs)
You may have heard of the rare blood clotting disorder, Antiphospholipid Syndrome, but did you know that it isn’t ‘just’ about the blood? This article is part of the Antiphospholipid Syndrome (APS) resource library that I’m building up on my site from a patient perspective. […]
Chronic voicesYou may have heard of the rare blood clotting disorder, Antiphospholipid Syndrome, but did you know that it isn’t ‘just’ about the blood? This article is part of the Antiphospholipid Syndrome (APS) resource library that I’m building up on my site from a patient perspective. It consists of findings from research journals, as well as over 20 years of my personal experiences with APS. This post will focus on how does Antiphospholipid Syndrome affect the body, beyond the blood to major organs.
I won’t deep dive into each medical condition, or the post will never end 
If there are terms or topics in this article that you’d like to learn more about, then check out the complete A – Z resource guide and related APS posts below as well!
Read the A – Z Antiphospholipid Syndrome Guide
*Disclaimer: This article is meant for educational purposes, and is based on my personal experiences as a patient. Whilst I have done my utmost to be meticulous in research, I am not a doctor, and nothing in this article should be substituted for medical advice. Please consult your own doctor before changing or adding any new treatment protocols. This post may also contain affiliate links. It will cost you nothing to click on them. I will get a small referral fee from purchases you make, which helps with the maintenance of this blog. Read our Privacy Policy page for more information. Thank you!
-
Changelog:
- 14 January 2025: Added new study by Huang et al. (2025) on future atherosclerotic cardiovascular disease in SLE/APS patients.
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Latest Research on Antiphospholipid Syndrome (2024 Edition)
- Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
- The Lowdown on Medications & Antiphospholipid Syndrome (Warfarin, Enoxaparin, DOACs, NSAIDs & More)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
Pin to Your Autoimmune Disease & Antiphospholipid Syndrome Boards:


Antiphospholipid Syndrome is a Systemic Autoimmune Disease
What this means simply (but we know it’s not that simple, is it?), is that it can affect any part of the body in different ways.
According to García-Carrasco et al. (2013):
“The clinical features and laboratory manifestations associated with aPL [antiphospholipid antibodies] have broadened considerably since the first description of APS in 1983 and now include thrombocytopenia, hemolytic anemia, cardiac valve disease, pulmonary hypertension, microangiopathic nephropathy, skin ulcers, livedo reticularis, refractory migraine, cognitive dysfunction, and atherosclerosis.”
Beyond direct blood-related manifestations, Antiphospholipid Syndrome can also affect the heart, lungs, skin, brain and more. People who have an autoimmune disease tend to have comorbidities as well (i.e. those mutations love a party). For one, researchers have found that there is a high risk of developing Systemic Lupus Erythematosus (SLE), especially during the first 5 years of being diagnosed with Antiphospholipid Syndrome (Chen et al., 2021).
Pin to Your Autoimmune Disease & Antiphospholipid Syndrome Boards:

Let’s Get the Bloody Issue Out of the Way First
Patients with Antiphospholipid Syndrome are at an increased risk of blood clots, due to the production of antiphospholipid antibodies (aPLs) that attack phospholipids (Cleveland Clinic, 2022e, August 19). They can cause abnormalities in white blood cells, red blood cells, platelets, and other components of blood both directly or indirectly. Symptoms of blood clots depend on the body part where they’re ‘stuck’ at. Let’s take a look at a few direct manifestations of Antiphospholipid Syndrome within the blood.
Deep Vein Thrombosis (DVT)
Venous thrombosis (VT) is the most common clinical manifestation of Antiphospholipid Syndrome, with up to 30% of APS patients having had an episode (Biggioggero and Meroni, 2010). A VT usually occurs as a deep vein thrombosis (DVT).
You’ve probably heard of DVTs (Reyes and Abe, 2023, May 1), in news articles scattered across the internet especially in relation to long-haul flights (Ward, 2024, April 5), as they can also occur in healthy people as a result of prolonged periods of inactivity. DVTs are blood clots that tend to form in a deep leg vein, although they can occur anywhere in the body. If these clots are large enough, they can also lodge in the brain, heart, lung or heart, which can quickly turn into a life-threatening situation.
-
Read Related Posts:
- Top Tips for Travelling with Chronic Illness & Disability (From a Girl Who Loves to Travel)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- My Recovery Time for Simultaneous Bilateral Patellar Tendon Rupture (With Lupus & Steroid Treatment)
- My Second Brush with Death: A Broken Heart (Literally)
- Bloody Mutations into Lupus
More About Veins
This article from Cleveland Clinic (2022c, June 19) does a fantastic job of explaining what veins are. To summarise, veins are blood vessels that are an important part of the circulatory system, and 75% of blood in the human body is contained within them.
Apart from the pulmonary veins, they carry oxygen-poor blood back to your heart (arteries carry oxygen-rich blood in reverse). Collectively, they form the venous system, and there are a few different kinds – deep veins, superficial veins and perforating veins. Deep veins are found in the muscles and along bones, and contain 90% of the blood that needs to make its way back to the heart in the legs. That is why it is more common to experience a DVT in the legs.
An interesting anatomy tidbit from Cleveland Clinic – calf muscles are also known as the ‘second heart’ as they help to pump blood back up against gravity. That is why it is important to keep moving and walking on long-haul flights, and why bedbound patients often need to wear compression stockings to prevent a DVT from occurring. Breathing is another important factor in helping blood to circulate.
Thrombocytopenia
Platelets are also known as thrombocytes, and thrombocytopenia is a condition when your platelet count is too low (National Heart, Lung, and Blood Institute, 2022, March 24). This can lead to excessive bleeding, as your blood is unable to clot sufficiently. Thrombocytopenia is also the most common non-criteria manifestation of antiphospholipid antibodies (aPLs), with a frequency of between 20% – 50% (Artim-Esen et al., 2015).
It is recognised as a complication of patients with lupus anticoagulant and anticardiolipin antibodies in particular. Those with APS and SLE concomitantly also exhibit thrombocytopenia with greater frequency. Having said that, thrombocytopenia is usually not severe in APS patients, and usually does not require therapy (Forastiero, 2012).
Pin to Your Health Boards:

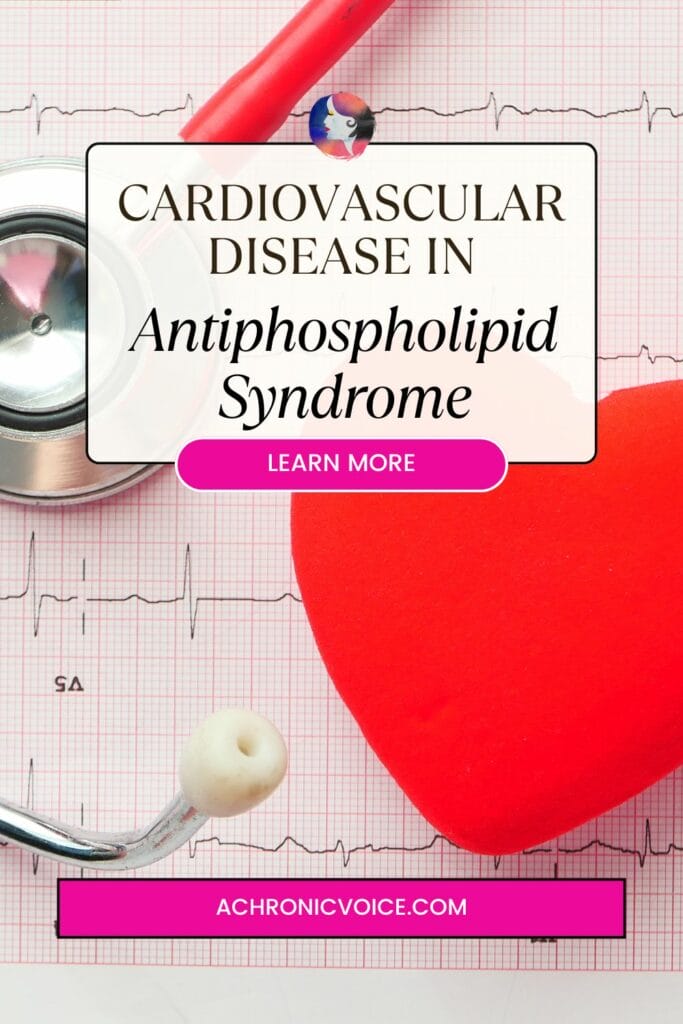
The Increased Risk of Cardiovascular Disease in Antiphospholipid Syndrome
Cardiovascular disease (CVD) is an umbrella term that covers disorders of the heart and blood vessels, and is the leading cause of death globally (World Health Organization, n.d.). The usual suspects contribute to CVD, such as smoking and an unhealthy diet. Smoking is also one of the most important predictable risk factors for cardiovascular disease in people with Antiphospholipid Syndrome. The combination is strongly associated with arterial vascular events, especially ischaemic strokes (when a blood clot blocks an artery in the brain) (Tektonidou, 2022; Mayo Clinic, n.d. -b). As such, it is important for people with APS to mediate what they can in an attempt to maintain and improve their overall health.
According to Polytarchou et al. (2020):
“Patients with APS have endothelial dysfunction, accelerated endothelial proliferation and intimal hyperplasia, atherogenesis, platelet activation, inflammatory products secretion and coagulation-fibrinolytic dysregulation.”
All that basically goes to say that APS patients are more prone to CVD, due to thrombotic and inflammatory factors in addition to traditional risk factors. Let’s break that down a bit to make more sense in relation to blood clotting and Antiphospholipid Syndrome.
What is the Endothelium?
Endothelium refers to the thin layer of cells that lines the inside of blood vessels, and endothelial cells secrete substances that control the opening and closing of arteries, which subsequently determines blood pressure and how hard your heart has to pump (Cleveland Clinic, 2022b, May 12). These substances also activate the fibrinolysis system (which helps to regulate blood clots) (MedLine Plus, n.d.), and mediates platelet adhesion and shear stress induced aggregation (Wu and Thiagarajan, 1996).
There are four different types of endothelial cells, namely: arterial, venous, capillary and lymphatic. These cells can be found in major organs, such as the brain, liver, kidney, lungs and heart, where they proliferate (reproduce) at different rates, serve different functions, and vary in movement (Przysinda et al., 2020).
Endothelial Dysfunction & Antiphospholipid Syndrome
According to Stanford Health Care (n.d. -b): “Endothelial dysfunction is a type of non-obstructive coronary artery disease (CAD) in which there are no heart artery blockages, but the large blood vessels on the heart’s surface constrict (narrow) instead of dilating (opening).” Endothelial dysfunction causes chronic chest pain, and is more frequently seen in women than in men.
This dysfunction is usually due to low levels of nitric oxide gas in blood vessel walls, which can trigger inflammation, and other platelet and blood vessel dysregulations. These can result in blood clots, strokes, hypertension, heart attacks, and more.
Patients with APS have been found to have impaired synthesis of nitric oxide that can be caused by various factors. APS patients with thrombosis were found to have low plasma levels of nitrites and nitrates, which are important metabolites for breaking down nitric oxide. Antiphospholipid antibodies also have implications in how nitric oxide synthesises (Velásquez et al., 2018).
Cardiovascular Diseases that APS Patients are More Prone to
According to Tektonidou (2022) and Polytarchou et al. (2020), a few of the types of cardiovascular diseases that patients with Antiphospholipid Syndrome are more prone to include:
- Hypertension (high blood pressure). Hypertension is a leading cause of death worldwide (World Health Organization, 2023, March 16). It is also one of the most common traditional risk factors for APS patients, where approximately 20 – 35% of APS patients have it. It is also more commonly found in patients with a combination of SLE/APS, as compared to PAPS (Primary Antiphospholipid Syndrome).
- Pulmonary Hypertension (PH). This is different from ‘regular’ hypertension, as it mainly affects either arteries or veins in the lungs; ‘regular’ hypertension on the other hand, is when there is constriction in your arteries, and can happen anywhere in the body (National Heart, Lung, and Blood Institute, 2023, May 1; Orlando Health, n.d.). People who are positive for antiphospholipid antibodies have been found to be susceptible to all five groups of PH, with those who have a comorbid connective tissue disorder such as Lupus, at a slightly higher risk.
- Hyperlipidemia (elevated lipids such as cholesterol). Hyperlipidemia is another prevalent medical condition, especially in developed countries that eat a high-fat, Western diet (Cleveland Clinic, 2022d, August 4). It is also another leading cause of cardiovascular disease in patients with Antiphospholipid Syndrome, and is present in about 20% – 25% of patients.
-
Atherosclerosis (hardening of arteries caused by a buildup of plaque). This is a prevalent condition, but many people may not be aware that they have it as they may not have symptoms in the early stages (Cleveland Clinic, 2024a, February 15). Studies have found that atherosclerotic plaques are associated with IgG and anti-β2GPI antibodies, and that APS and/or SLE/APS patients had almost 2.5 fold the risk of them developing in the carotid and/or femoral arteries.
Another recent study by Huang et al. (2025) also concluded that “SLE patients with positive aPLs, especially positive aCL [anticardiolipin] IgG/IgM and LA [lupus anticoagulant], warrant more care and surveillance of future ASCVD [atherosclerotic cardiovascular disease] events during follow-up”. The study also found that aCL IgA and anti-β2GPI IgA antibodies were independent risk factors for ASCVD, even in participants without autoimmune disease.
-
Acute Coronary Syndromes (ACS). These are a group of disorders that include heart attacks and unstable anginas (chest pain when your heart muscle doesn’t get enough oxygen-rich blood), and is a medical emergency when it happens. ACS most commonly happens due to a plaque bursting, or when a blood clot blocks blood flow to the heart (Cleveland Clinic, 2022a, May 2; Cleveland Clinic, 2024c, May 24).
Patients with Antiphospholipid Syndrome have an increased risk of ACS, which can happen even with normal or non-obstructive coronary artery disease, or with normal or near-normal coronary arteries (Stanford Health Care, n.d -a). Acute myocardial infarctions (heart attacks) that happen to young patients, especially those in their forties, can often be attributed to Antiphospholipid Syndrome (Nevras et al., 2023). There could be a few possible reasons as to why APS patients are more prone to ACS, such as plaque ruptures or an acute thrombosis event.
-
Valvular Heart Disease. This happens when any valve in the heart is damaged or diseased, with the most common being stenosis, where the valve becomes narrow or stiff, and thus unable to open fully to allow blood to flow through (CDC, 2024, May 31).
Libman-Sacks endocarditis is present in 30% of APS patients, and according to Polytarchou et al. (2020):
“Typically, patients with APS have valve thickening (>3 mm) of the proximal or middle portion of the leaflets, or irregular nodules on the atrial aspect of the edge of the mitral valve or the vascular surface of the edge of the aortic valve. The formation of valve vegetation, known as Libman-Sacks endocarditis, is the result of endocardial damage and thrombus formation.”
I personally have had a mitral valve prolapse when I was 25, though I don’t think it can be solely attributed to APS as I have a whole assortment of chronic illnesses. I still remember slowly dying over the course of a year, as breathing became increasingly difficult. I managed to eventually get it repaired at Cleveland Clinic, as the local surgeons were not keen to touch a patient with Antiphospholipid Syndrome. That life-saving surgery was only made possible thanks to the hundreds of kind, generous souls who funded it.
- Cardiomyopathy (heart muscle disorders). There are various types of conditions that can cause cardiomyopathy, which results in your heart being unable to pump blood efficiently. Over time, this weakens your heart and can lead to heart failure. APS patients, especially those with a comorbid SLE diagnosis, have an increased risk of cardiomyopathy, most likely due to “microvascular thrombosis, autoimmune vasculitis and myocarditis or microvascular fibrosis” (Polytarchou et al., 2020).
- Intracardiac Thrombi. Cardiac thrombi can be commonly found in patients with ischaemic strokes, and it is important to distinguish them from other cardiac masses such as tumours, in order to render proper treatment (Alkindi et al., 2013). Intracardiac thrombosis is when a blood clot forms in the heart, which can also lead to pulmonary or peripheral embolisms.
This is not an exhaustive list of cardiological issues in relation to Antiphospholipid Syndrome, but I hope that it has granted you some insight into the mechanisms behind it, and that it serves to highlight the importance of maintaining good heart wherever possible (I personally could do better, for one…).
Pin to Your Cardiovascular Health & Antiphospholipid Syndrome Boards:
Cutaneous Manifestations in Antiphospholipid Syndrome
Cutaneous (skin-related) manifestations are common and may actually be the first signs of Antiphospholipid Syndrome. In a study of 200 patients, Francès et al. (2005) found that 49% of APS or APS/SLE patients had dermatologic manifestations, and that it was the presenting manifestation in 30.5% of them. Kriseman et al. (2007) also notes that 40% of APS patients who have cutaneous manifestations go on to develop multisystemic thrombotic events, which underscores the need to be extra vigilant.
According to Gibson et al. (1997), cutaneous manifestations in Antiphospholipid Syndrome include:
“livedo reticularis, necrotizing vasculitis, livedoid vasculitis, thrombophlebitis, cutaneous ulceration and necrosis, erythematous macules, purpura, ecchymoses, painful skin nodules, and subungual splinter hemorrhages.”
Studies have shown that livedo reticularis is the most common dermatologic manifestation in APS patients, at about 55%. Another study of 70 patients with the lupus anticoagulant showed thrombophlebitis as the most common at about 34%. SLE is often associated with secondary cases, and chronic urticaria is also associated with autoimmune conditions in general, in approximately 50% of cases (Diógenes et al., 2004).
Let’s take a look at how Antiphospholipid Syndrome can affect the largest organ of the human body – the skin.
Livedo – The Most Common Dermatologic Manifestation in APS Patients
Livedo reticularis is a netlike, purplish discolouration of skin, thought to be caused through the constriction of blood vessels (Danan et al., 2021, January 19). This then disrupts blood flow, and results in oxygen-starved red blood cells accumulated beneath the skin. It may also be the presenting sign of APS, although it may be hard to tell as it can also occur in other individuals with or without other medical conditions.
There is also another form of livedo known as livedo racemosa, which presents as a discontinued network, and does not go away. It mostly occurs in the lower limbs, and for APS patients, usually happens due to organised thrombosis. Unlike livedo reticularis, livedo racemosa is commonly associated with thrombotic or inflammatory disorders (Pincelli et al., 2021).
Superficial Thrombophlebitis
Superficial thrombophlebitis is when there is inflammation of the veins just beneath your skin, and usually occurs in the legs. Symptoms include swelling, redness or tenderness, and sometimes a high fever (NHS, 2022, June 20). These are similar to that of a DVT, but with less severity. A small study of 45 patients with recurrent superficial thrombophlebitis also found a correlation with anticardiolipin antibodies (de Godoy et al., 2001).
Cutaneous Ulceration
According to Dobler et al. (2018), about 20% – 30% of Antiphospholipid Syndrome patients have lower extremity ulcers, which they posit might be due to “vascular endothelial damage at the microcirculation level, leading to intracapillary thrombosis and focal inflammation”.
Whilst the full pathology is yet to be fully understood, some recent studies have suggested that the antiphospholipid antibodies – lupus anticoagulant and anticardiolipin – might be risk factors for venous leg ulcers, which might cause repeated thrombosis that lead to chronic damage that are unable to heal properly over time (Takahashi et al., 2021).
Research I have found in relation to skin ulcers are mostly from case reports, where many of the ulcers resembled pyoderma gangrenosum, which is an ulcerative disorder that is not fully understood, but commonly linked with systemic diseases (Schmieder and Krishnamurthy, 2023, July 4). These ulcers either fully resolved with a combination of treatments including anticoagulation, although there was one case report of an ulcer that wasn’t able to heal for 7 years (Wei et al., 2022). In all of the case reports, the need for a multidisciplinary approach was emphasised.
Pin to Your Skin & Antiphospholipid Syndrome Boards:

Male & Female Reproductive Systems
I wrote a whole post about females and Antiphospholipid Syndrome which you can read about in the link below (and will follow up with one all about males at some point!). If you do not have the time, one thing to be aware of are ovarian cyst ruptures, which can come on suddenly, and is of life-threatening status. Having experienced it twice, I am now on birth control in a bid to prevent it from happening again.
Whether Antiphospholipid Syndrome contributes to infertility is still controversial, as there is insufficient data to conclude. However, there have been case reports of testicular thrombosis followed by orchiectomy (surgical procedure to remove testicle(s)) in males with APS, which could contribute to future infertility (El Hasbani et al., 2020).
-
Read Related Posts:
- Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
- What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
- YuYu Bottle Review: Hot Water Bottle for ‘Surround Warmth’ Pain Relief
Musculoskeletal Manifestations in Antiphospholipid Syndrome
Several musculoskeletal manifestations have been reported in APS patients, namely: Arthralgia/Arthritis, Avascular Necrosis/Osteonecrosis, bone marrow necrosis, complex regional pain syndrome type-1 (reflex sympathetic dystrophy), muscle infarction, non-traumatic fractures and osteoporosis (Noureldine et al., 2016). Musculoskeletal manifestations can also be complicated by comorbidities, such as Lupus (SLE).
Patients on long-term warfarin therapy can also lose bone density as it is a Vitamin K antagonist, which is an important vitamin for bone health (Rodríguez-Olleros Rodríguez and Díaz Curiel, 2019). Learn more about Vitamin K antagonists here.
-
Read Related Posts:
- Vitamin D & Vitamin K2: How They Boost Each Other in the Body
- Oral Spray Vitamins: A Quick & Easy Way to Get Your Nutrients with Chronic Illness
- What It Feels Like to be Suddenly Disabled
- My Recovery Time for Simultaneous Bilateral Patellar Tendon Rupture (With Lupus & Steroid Treatment)
- What’s it Like to be on a High Dose of Steroids? (And the First Question You Will Definitely Ask)
Avascular Necrosis / Osteonecrosis
Avascular necrosis of bone (AVN) is also known as osteonecrosis (ON) or aseptic necrosis. It is a disease in which cell death occurs in components of bone, as a result of interruption in blood supply. AVN is associated with several autoimmune diseases.
For Antiphospholipid Syndrome, ischaemia is thought to be the main cause, with antiphospholipid antibodies associated with vessel thromboses. Thrombosis of terminal arteries in the subcondral areas (layers of bone just beneath the cartilage in a joint) has also been found in patients with non-traumatic AVN (Tektonidou and Moutsopoulos, 2006). Studies have shown that previous glucocorticoid (steroid) use and thrombocytopenia (low platelet count) may be contributing factors (Freire de Carvalho et al., 2021).
Osteopenia & Osteoporosis
Osteopenia refers to bone density loss, which can lead to osteoporosis where the bones have become weak and brittle, and thus can break easily (Cleveland Clinic, 2024b, March 19; Mayo Clinic, n.d. -a).
Long-term warfarin use has been associated with osteoporosis, especially for men. This might be due to its Vitamin K antagonistic effects, which interferes with bone formation (Gage et al., 2006). Another small study also showed a strong correlation between antiphospholipid antibodies and metatarsal fractures (which includes osteoporosis), although the role of warfarin is yet unclear (Sangle et al., 2004).
Learn more about warfarin here.
Arthralgia
As per Noureldine et al.’s (2016) review, Primary Antiphospholipid Syndrome (PAPS) is a common cause of arthralgia (pain in a joint). This is different from Arthritis, which is an actual diagnosis, and not a symptom (Hardin, 1990). Management of arthralgia and arthritis is primarily on a symptomatic basis, with drugs such as NSAIDs and analgesics.
According to Noureldine et al. (2016), osteoarticular pain might be due to a flare for SLE-APS patients, in which immunosuppressive agents and/or corticosteroids may be needed. I have both Sjögren’s and SLE, and based on my personal experience, only steroids work when I’m in an immense pain flare. Even strong painkillers do nothing to ease the pain.
-
Read Related Posts:
- Why Painkillers are One of My Biggest Allies for a Decent Quality of Life
- What It Feels Like to be Suddenly Disabled
- “But That’s Normal for Me” (Why I Mistook Dengue Fever for a Lupus Flare)
- 12 Visible Evidence of a Body Gone Rogue (Is Invisible Illness Truly Invisible?)
- 3 Types of Chronic Pain that Sound Bearable, but are Not
Pin to Your Antiphospholipid Syndrome Awareness Boards:

Neuropsychiatric Manifestations in Antiphospholipid Syndrome
Neuropsychiatry is a field of medicine which involves neurology and also mental illness (Royal College of Psychiatrists, n.d.). Antiphospholipid Syndrome is now recognised as a major neurological disease as well. Neurological events that can occur due to APS include: stroke, transient ischemic attacks (TIA), migraine, headaches, memory loss, ataxia (coordination/balance issues), symptoms that mimic Multiple Sclerosis, myelopathy, neuropathy, behavioural disorders and more (Hughes, 2003; Johns Hopkins Medicine, n.d. -a; Penn Medicine, n.d.; Healthdirect Australia, 2022, September).
The direct impact of antiphospholipid antibodies (aPLs) on the central nervous system (CNS) have also been postulated to explain the effect of neurological symptoms in APS patients. One study showed that aPLs bound to specific areas of the brain that impacted memory and learning functions. Another study associated long-term exposure to apLs with motor hypoactivity and impaired cognition, due to mature amyloid plaque deposition, and a relationship between thrombin and coagulation inhibitors. This could potentially increase the risk of Alzheimer’s Disease. The increased exposure to proinflammatory cytokines most likely play a role as well (Carecchio et al., 2014).
A lot of research still needs to be done in terms of APS and neuropsychiatric manifestations, as the mechanisms are yet to be fully understood. This is also a great video by Dr. Sanil Rege, who explains more about neuropsychiatric manifestations of APS in a simple manner (Psych Scene Hub, 2024, April 26), and another one on the subject by Prof. Graham Hughes (Psych Scene Hub, 2020, August 11).
Brain Fog / Cognitive Dysfunction
An impact in cognitive function is preferable to saying ‘brain fog’, which often makes the experience sound too trivial. Those who live with ‘brain fog’ know how devastating its impacts are. Cognitive dysfunction is also another annoying feature of APS involvement in the neurological pathways, and exists on a spectrum from mild to severe (such as dementia).
The frequency of cognitive dysfunction ranges from 19% to 40%, and includes cognitive complication with memory, executive function, visuospatial skills and visuomotor speed. APS patients can also present with psychiatric symptoms such as: psychosis, mania, depression, bipolar disorders, OCD and schizophrenia (Yelnik et al., 2016). Here is also a useful Q&A session with Dr. Yu, as he answers some questions from the APS community regarding brain fog (Yu, n.d.).
Combined with Lupus, Epilepsy, Sjögren’s disease, depression, anxiety and all the other medical conditions that I have, nailing down the culprit of my brain fog can be tricky. I often think that brain fog can be worse than pain, because at least there are coping strategies for pain to a certain extent. There is not much you can do for brain fog, where I have trouble adding 3 + 2, even. You can see how that’s detrimental to trying to get anything done, from simple chores to work.
-
Read Related Posts:
- Sometimes, Physical Pain Isn’t the Worst Part About Chronic Illness
- So This is What a Tonic Clonic Seizure Feels Like
- Today is Not a Good Day to Make Decisions (and That’s Okay)
- 12 Visible Evidence of a Body Gone Rogue (Is Invisible Illness Truly Invisible?)
- The Savagery of Panic Attacks & The Saving Grace of Internet Friends
Stroke / Cerebrovascular Accident
A stroke is known medically as a cerebrovascular accident (State of Hawaii, Department of Health, n.d.), and is one of the big bad ones when Antiphospholipid Syndrome misbehaves. Apart from taking your medications fastidiously, steps for stroke prevention require modifications to your lifestyle. This includes eating a balanced diet (especially if you’re on warfarin), the avoidance of contact sports, and more. Basically, things you probably would not have thought about twice before your Antiphospholipid Syndrome diagnosis.
Studies have shown that triple positive APS patients are at the highest risk for thrombosis, whilst other studies have shown that patients with SLE with only lupus anticoagulant are at an equally high risk.
According to Mittal et al. (2023):
“Acute ischemic stroke (AIS) and transient ischemic attack (TIA) are the most common manifestations of arterial pathology in APS,7 with approximately 20% of patients with APS suffering a stroke more than 10 years.8 In individuals aged below 50 years, 17% of strokes and 12% of TIA are associated with aPL,9 suggesting APS is an important cause of strokes in younger patients.”
Another important thing to note is that more than 20% of strokes in patients younger than 45 years of age may be attributed to Antiphospholipid Syndrome (Ricarte et al., 2018). It is also more common in males.
Transient Ischaemic Attack (TIA)
A transient ischaemic attack (TIA) is a ‘mild stroke’ event that doesn’t last for long, and occurs when blood supply to the brain is cut off briefly. Symptoms include: numbness especially on one side of the body, confusion, vision problems, dizziness, and loss of coordination in articulation and balance (National Institute of Neurological Disorders and Stroke, n.d.). I also know a friend with Lupus (SLE) in her mid-30s, who was recently diagnosed with APS, as she had suffered a stroke with atypical symptoms.
Strokes and transient ischaemic attacks (TIAs) are the most common neuropsychiatric manifestations of Antiphospholipid Syndrome. A TIA was actually my first manifestation and experience with APS at 14, where exactly half of my body was numb. It was a strange sensation, almost as if I were sliced into half with precision. I brushed it off as a heat stroke, as I had just endured a physical training session under the hot sun. I was still joking with my friend as we dragged my body to a general practitioner, who referred me to a neurologist. I then received an Antiphospholipid Syndrome diagnosis, and I can still remember that devastating day with clarity, even 20 years on.
Pin to Your Autoimmunity & Antiphospholipid Syndrome Boards:

Ophthalmologic / Ocular Manifestations in Antiphospholipid Syndrome
Ophthalmology is a field in medicine with many sub-specialties, and deals with the eyes and vision, their functions and diseases (Churchill and Gudgel, 2024, February 24). It is important to note that thrombosis can occur in the eyes as well. Apart from antiphospholipid antibodies (aPLs), proinflammatory cytokines also play a role in triggering thrombosis.
As usual, comorbidities such as SLE can worsen things; according to Neto et al. (2023), up to 1/4 of APS/SLE and SLE patients had retinal abnormalities, and the presence of aPL triple positivity and a high aGAPSS score also seem to be risk factors for paracentral acute middle maculopathy (a type of ischemic maculopathy) (Mishra et al., 2023).
There are a number of ocular and neuroophthalmic manifestations that have been found in APS patients, including but not limited to: retinal arteritis, retinal venous occlusion, ischemic optic neuropathy, transient loss of vision and diplopia (Suvajac et al., 2007). Both anterior and posterior eye segments can be affected (Franco et al., 2020), and sometimes symptoms overlap with neurological-type manifestations such as headache and migraine-like visual symptoms (Uludag et al., 2021).
According to Suvajac et al. (2007), the most frequent ocular manifestation in APS is retinal thrombosis, especially in young adults. In secondary APS, occlusion of central retinal artery and vein (OACR, OVCR) is the most common finding. Patients with Lupus (SLE) on top of Antiphospholipid Syndrome have a compounding of ophthalmic issues, such as scleritis, extraocular thromboses, and optic neuropathy. Catastrophic APS (CAPS) is rare but can also affect the eyes, which can even lead to permanent vision loss (Morel et al., 2021).
Ocular and ophthalmic manifestations in Antiphospholipid Syndrome was previously thought to be rare, but further studies have revealed that they actually occur in 15 – 88% of patients (Suvajac et al., 2007). Interestingly, risk factors associated with retinal vasculopathy include APS-related kidney and heart valve disease, as well as obstetric morbidity (Xie et al., 2022).
Retinal Venous Occlusion
Retinal vein occlusion (RVO) is a multifactorial retinal vascular disease that can cause vision damage and blindness, and is common amongst the elderly. Antiphospholipid antibodies are also a risk factor for RVO, especially in patients less than 45 years of age (Zhu et al., 2015). In a study by Hernández et al. (2020), it was found that 10% of the patients with RVO had antiphospholipid antibodies, and that up to 90% of their RVO-APS patients had at least one vascular risk factor.
Pulmonary Manifestations in Antiphospholipid Syndrome
Pulmonary thromboembolisms and pulmonary hypertension are the most common manifestations of APS in the lungs. Sometimes, patients get a pulmonary embolism first, which leads to a diagnosis of Antiphospholipid Syndrome.
Other APS and lung issues include, but are not limited to: microvascular pulmonary thrombosis, pulmonary capillaritis, alveolar haemorrhage, acute respiratory distress syndrome (ARDS) and postpartum syndrome (Espinosa et al., 2002).
Pulmonary Embolism (PE)
A Pulmonary Embolism (PE) is when a blood clot develops in one of your veins (often in the legs), and travels to lodge itself in a lung artery, blocking blood flow (Johns Hopkins Medicine, n.d. -c). There is a high mortality rate for PEs, with Antiphospholipid Syndrome as a risk factor. According to Shi et al.( 2022) in a study of 76 patients with PE:
“The risk factors for APS in PE patients are male, low PLT, prolonged APTT and slightly increased D-dimer.”
Many people who live with all types of chronic illnesses or disabilities tend to “wait for a bit and see”. Going to the A&E is not fun – I don’t think I need to explain why. It is also uncomfortable, full of other sick people, and all that waiting around makes you even more dehydrated and exhausted. “Is it worth a trip? The pain isn’t so bad…yet…right?”, you think to yourself. And I’ve had that thought many times.
But if you have breathlessness or any persistent chest pains that will not go away, especially where painkillers don’t even help – then please just go to the A&E. A pulmonary embolism can be deadly, and the longer you wait, the more damage it will cause.
You can read about my personal experience with Pulmonary Embolism in this post. This medical incident put my body under huge physical and mental stress, which subsequently activated all the other genes for Lupus, Sjögren’s and more, which might have otherwise remained dormant.
Pin to Your Anatomy, Health & Antiphospholipid Syndrome Boards:
Vascular Manifestations in Antiphospholipid Syndrome
The vascular system is also known as the circulatory system. It consists of the blood vessels (arteries and veins), capillaries (tiny arteries between blood vessels), and lymph vessels. Its functions include blood circulation and lymphatic drainage. These have overlaps with the respiratory, digestive, kidney and urinary system, as well as temperature control, as they all rely on the vascular system (Johns Hopkins Medicine, n.d. -b). This also means that vascular manifestations of Antiphospholipid Syndrome can happen within any of these pathways.
There is increasing evidence that activation of the mammalian target of rapamycin complex (mTORC) pathway by antiphospholipid antibodies (aPLs) is associated with vascular lesions (Canaud et al., 2014). In a study of 48 APS patients, it was also found that those with increased TLR-2 and TLR-4 (toll-like receptor proteins) had endothelial dysfunction, arterial stiffening, and hypertrophy (Benhamou et al., 2014).
No matter the organ involved, most of these vascular manifestations occur as acute or chronic lesions and/or thrombosis in various forms. These can subsequently lead to more specific medical issues, such as stenotic/occlusive coronary arterial disease in the heart, “APS nephropathy” in the kidneys, on top of a myriad of other vascular-related diseases. It is also important to note, once again, that comorbidities such as Lupus (SLE) compounds these issues, due to a wide variety of added factors (Siddique et al., 2017).
Diffuse Alveolar Haemorrhage
Diffuse Alveolar Haemorrhage (DAH) is a small vessel vasculitis that damages the lung microvasculature, so the most fatal complication often involves the respiratory system (Stoots et al., 2019). This is a rare condition that can happen to APS patients, with a high mortality rate between 30.3% – 45.8%.
Symptoms of diffuse alveolar haemorrhage include: dyspnea (shortness of breath), cough, hypoxemia (low blood oxygen levels), hemoptysis (coughing blood out from the lungs), fever, and more. Treatment is usually fairly aggressive, and include: glucocorticoids, immunosuppressive therapy, plasma exchange, and more. More than half of APS patients with DAH are estimated to have a relapse within 5 years of follow-up (Figueroa-Parra et al., 2023).
Conclusion: How Does Antiphospholipid Syndrome Affect the Body?
As you can see, Antiphospholipid Syndrome is a systemic autoimmune disease that goes beyond its status as a ‘mere’ blood disorder. Blood is life, and blood clots can occur in any part of your body, leading to potentially detrimental effects. Thus, it is critical to take your APS diagnosis seriously. Learn from my mistakes – sticking to a regular balanced diet and avoiding contact sports are some things you can do to help lessen the risk of a severe incident. You may not be experiencing pain in the present moment, but remember that prevention is better than cure.
If you liked this article and found it helpful, you can support me via the button below (no obligations, though!) 
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Antiphospholipid Syndrome Diagnosis: The A to Z Guide as a Patient
- Latest Research on Antiphospholipid Syndrome (2024 Edition)
- Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
- The Lowdown on Medications & Antiphospholipid Syndrome (Warfarin, Enoxaparin, DOACs, NSAIDs & More)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
Pin to Your Antiphospholipid Syndrome & Chronic Illness Boards:


-
References:
- Alkindi, F., Hamada, A. H. S., & Hajar, R. (2013). Cardiac Thrombi in Different Clinical Scenarios. Heart Views : The Official Journal of the Gulf Heart Association, 14(3), 101–105. https://doi.org/10.4103/1995-705X.125924
- Artim-Esen, B., Diz-Küçükkaya, R., & İnanç, M. (2015). The Significance and Management of Thrombocytopenia in Antiphospholipid Syndrome. Current Rheumatology Reports, 17(3), 14. https://doi.org/10.1007/s11926-014-0494-8
- Biggioggero, M., & Meroni, P. L. (2010). The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmunity Reviews, 9(5), A299–A304. https://doi.org/10.1016/j.autrev.2009.11.013
- Carecchio, M., Cantello, R., & Comi, C. (2014). Revisiting the Molecular Mechanism of Neurological Manifestations in Antiphospholipid Syndrome: Beyond Vascular Damage. Journal of Immunology Research, 2014(1), 239398. https://doi.org/10.1155/2014/239398
- CDC. (2024, May 31). About Valvular Heart Disease. CDC. Retrieved from: https://www.cdc.gov/heart-disease/about/valvular-heart-disease.html
- Chen, H. H., Lin, C. H., & Chao, W. C. (2021). Risk of systemic lupus erythematosus in patients with anti-phospholipid syndrome: a population-based study. Frontiers in medicine, 8, 654791. https://doi.org/10.3389/fmed.2021.654791
- Churchill, J., & Gudgel, D. T. (2024, February 24). What Is an Ophthalmologist vs Optometrist? American Academy of Ophthalmology. Retrieved from: https://www.aao.org/eye-health/tips-prevention/what-is-ophthalmologist
- Cleveland Clinic. (2022a, May 2). Acute Coronary Syndrome. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/22910-acute-coronary-syndrome
- Cleveland Clinic. (2022b, May 12). Endothelial Dysfunction. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/23230-endothelial-dysfunction
- Cleveland Clinic. (2022c, June 19). Veins. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/body/23360-veins
- Cleveland Clinic. (2022d, August 4). Hyperlipidemia. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/21656-hyperlipidemia
- Cleveland Clinic. (2022e, August 19). Antiphospholipid Syndrome. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/21685-antiphospholipid-syndrome
- Cleveland Clinic. (2024a, February 15). Atherosclerosis. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/16753-atherosclerosis-arterial-disease
- Cleveland Clinic. (2024b, March 19). Osteopenia. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/21855-osteopenia
- Cleveland Clinic. (2024c, May 24). Unstable Angina. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/21744-unstable-angina
- Danan, V., Harp, J., & Erkan, D. (2021, January 19). Antiphospholipid Syndrome and Skin Problems – Top 10 Series. Hospital for Special Surgery. Retrieved from: https://www.hss.edu/conditions_top-ten-antiphospholipid-syndrome-skin-problems.asp
- Dobler, C., Szeyko, L. A., Landeros, M. M., Arango, C. P., Noble, L. S., Tano, S. V., & Herrada, J. (2018). Resolution of Intractable Leg Ulcers Associated with Antiphospholipid Syndrome (APS) with Prophylactic Dose of Aspirin (ASA) and Enoxaparin: A Case Report. Blood, 132, 5071. https://doi.org/10.1182/blood-2018-99-110752
- El Hasbani, G., Khamashta, M., & Uthman, I. (2020). Antiphospholipid syndrome and infertility. Lupus, 29(2), 105–117. https://doi.org/10.1177/0961203319893763
- Espinosa, G., Cervera, R., Font, J., & Asherson, R. A. (2002). The lung in the antiphospholipid syndrome. Annals of the Rheumatic Diseases, 61(3), 195–198. https://doi.org/10.1136/ard.61.3.195
- Figueroa-Parra, G., Meade-Aguilar, J. A., Langenfeld, H. E., González-Treviño, M., Hocaoglu, M., Hanson, A. C., Prokop, L. J., Murad, M. H., Cartin-Ceba, R., Specks, U., Majithia, V., Crowson, C. S., & Duarte-García, A. (2023). Clinical features, risk factors, and outcomes of diffuse alveolar hemorrhage in antiphospholipid syndrome: A mixed-method approach combining a multicenter cohort with a systematic literature review. Clinical Immunology, 256, 109775. https://doi.org/10.1016/j.clim.2023.109775
- Forastiero, R. (2012). Bleeding in the antiphospholipid syndrome. Hematology, 17(sup1), s153–s155. https://doi.org/10.1179/102453312X13336169156654
- Francès, C., Niang, S., Laffitte, E., Pelletier, F. le, Costedoat, N., & Piette, J. C. (2005). Dermatologic manifestations of the antiphospholipid syndrome: Two hundred consecutive cases. Arthritis & Rheumatism, 52(6), 1785–1793. https://doi.org/10.1002/art.21041
- Franco, A. M. de M., Medina, F. M. C., Balbi, G. G. M., Levy, R. A., & Signorelli, F. (2020). Ophthalmologic manifestations in primary antiphospholipid syndrome patients: A cross-sectional analysis of a primary antiphospholipid syndrome cohort (APS-Rio) and systematic review of the literature. Lupus, 29(12), 1528–1543. https://doi.org/10.1177/0961203320949667
- Freire de Carvalho, J., Correia de Araujo, R. P., & Skare, T. L. (2021). Osteonecrosis in Primary Antiphospholipid Syndrome is Associated with Previous Glucocorticoid Use and Thrombocytopenia. Rheumatology and Therapy, 8(3), 1255–1261. https://doi.org/10.1007/s40744-021-00333-9
- Gage, B. F., Birman-Deych, E., Radford, M. J., Nilasena, D. S., & Binder, E. F. (2006). Risk of Osteoporotic Fracture in Elderly Patients Taking Warfarin: Results From the National Registry of Atrial Fibrillation 2. Archives of Internal Medicine, 166(2), 241–246. https://doi.org/10.1001/archinte.166.2.241
- García-Carrasco, M., Pinto, C. M., Hernández, C. J., Poblano, J. C. S., Morales, I. E., & Martínez, S. M. (2013). Antiphospholipid syndrome. In Autoimmunity: From Bench to Bedside [Internet]. El Rosario University Press. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK459442/
- Gibson, G. E., Daniel Su, W. P., & Pittelkow, M. R. (1997). Antiphospholipid syndrome and the skin. Journal of the American Academy of Dermatology, 36(6), 970–982. https://doi.org/10.1016/S0190-9622(97)80283-6
- Hardin, J. G. (1990). Arthralgia. In H. K. Walker, W. D. Hall, & J. W. Hurst (Eds.), Clinical Methods: The History, Physical, and Laboratory Examinations (3rd ed.). Butterworths. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK303/
- Healthdirect Australia. (2022, September). Neuropathy. Healthdirect Australia. Retrieved from: https://www.healthdirect.gov.au/neuropathy
- Hernández, J. L., Sanlés, I., Pérez-Montes, R., Martínez-Taboada, V. M., Olmos, J. M., Salmón, Z., Sierra, I., Escalante, E., & Napal, J. J. (2020). Antiphospholipid syndrome and antiphospholipid antibody profile in patients with retinal vein occlusion. Thrombosis Research, 190, 63–68. https://doi.org/10.1016/j.thromres.2020.04.005
- Huang, C., Ding, Y., Chen, Z., Wu, L., Wei, W., Zhao, C., Yang, M., Lin, S., Wang, Q., Tian, X., Zhao, J., Li, M., & Zeng, X. (2025). Future atherosclerotic cardiovascular disease in systemic lupus erythematosus based on CSTAR (XXVIII): The effect of different antiphospholipid antibodies isotypes. BMC Medicine, 23(1), 8. https://doi.org/10.1186/s12916-024-03843-9
- Hughes, G. R. V. (2003). Migraine, memory loss, and “multiple sclerosis ”. Neurological features of the antiphospholipid (Hughes’) syndrome. Postgraduate Medical Journal, 79(928), 81–83. https://doi.org/10.1136/pmj.79.928.81
- Johns Hopkins Medicine. (n.d. -a). Ataxia. Johns Hopkins Medicine. Retrieved 2024, July 1, from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/ataxia
- Johns Hopkins Medicine. (n.d. -b). Overview of the Vascular System. Johns Hopkins Medicine. Retrieved 2024, July 1, from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/overview-of-the-vascular-system
- Johns Hopkins Medicine. (n.d. -c). Pulmonary Embolism. Johns Hopkins Medicine. Retrieved 2024, July 1, from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/pulmonary-embolism
- Kriseman, Y. L., Nash, J. W., & Hsu, S. (2007). Criteria for the diagnosis of antiphospholipid syndrome in patients presenting with dermatologic symptoms. Journal of the American Academy of Dermatology, 57(1), 112–115. https://doi.org/10.1016/j.jaad.2006.11.033
- Mayo Clinic. (n.d. -a). Osteoporosis. Mayo Clinic. Retrieved 2024, July 1, from: https://www.mayoclinic.org/diseases-conditions/osteoporosis/symptoms-causes/syc-20351968
- Mayo Clinic. (n.d. -b). Stroke. Mayo Clinic. Retrieved 2024, July 1, from: https://www.mayoclinic.org/diseases-conditions/stroke/symptoms-causes/syc-20350113
- MedLine Plus. (n.d.). Fibrinolysis – primary or secondary. National Library of Medicine. Retrieved 2024, July 1, from https://medlineplus.gov/ency/article/000577.htm
- Mishra, P., Mohanty, S., Shanmugasundaram, P., Moharana, B., & Das, D. (2023). Paracentral Acute Middle Maculopathy As the Presenting Sign of Ischemic Cardiomyopathy. Cureus, 15(2). https://doi.org/10.7759/cureus.35418
- Mittal, P., Quattrocchi, G., Tohidi-Esfahani, I., Sayar, Z., Chandratheva, A., & Cohen, H. (2023). Antiphospholipid syndrome, antiphospholipid antibodies, and stroke. International Journal of Stroke, 18(4), 383–391. https://doi.org/10.1177/17474930221150349
- Morel, N., Bonnet, C., Mehawej, H., Le Guern, V., Pérard, L., Roumier, M., Brezin, A., Godeau, B., Haroche, J., Benhamou, Y., Lambert, M., Yelnik, C. M., Maillard, N., Bodaghi, B., Piette, J.-C., & Costedoat-Chalumeau, N. (2021). Catastrophic Antiphospholipid Syndrome And Posterior Ocular Involvement: Case Series of 11 Patients and Literature Review. Retina, 41(11), 2332. https://doi.org/10.1097/IAE.0000000000003185
- National Heart, Lung, and Blood Institute. (2023, May 1). Pulmonary Hypertension – What Is Pulmonary Hypertension? National Institutes of Health. Retrieved from: https://www.nhlbi.nih.gov/health/pulmonary-hypertension
- National Heart, Lung, and Blood Institute. (2022, March 24). Platelet Disorders — Thrombocytopenia. National Institutes of Health. Retrieved from: https://www.nhlbi.nih.gov/health/thrombocytopenia
- National Institute of Neurological Disorders and Stroke. (n.d.). Transient Ischemic Attack (TIA). National Institutes of Health. Retrieved 2024, July 1, from:https://www.ninds.nih.gov/health-information/disorders/transient-ischemic-attack-tia
- Neto, E. D. S., Neto, T. S. R., Signorelli, F., Balbi, G. G. M., Higashi, A. H., Monteiro, M. L. R., Bonfá, E., Andrade, D. C. O., & Zacharias, L. C. (2023). Ocular retinal findings in asymptomatic patients with antiphospholipid syndrome secondary to systemic lupus erythematosus. Clinical Rheumatology, 42(8), 2105–2114. https://doi.org/10.1007/s10067-023-06613-9
- Nevras, V., Milaras, N., Katsioulis, C., Sotiriou, Z., Tsalamandris, S., Gkounti, G., & Skevos, S. (2023). Acute Coronary Syndromes in Antiphospholipid Syndrome-above Suspicion: A Systematic Review. Current Problems in Cardiology, 48(3), 101503. https://doi.org/10.1016/j.cpcardiol.2022.101503
- NHS. (2022, June 20). Symptoms – Antiphospholipid syndrome (APS). NHS. Retrieved from: https://www.nhs.uk/conditions/antiphospholipid-syndrome/symptoms/
- Noureldine, M. H. A., Khamashta, M. A., Merashli, M., Sabbouh, T., Hughes, G. R. V., & Uthman, I. (2016). Musculoskeletal manifestations of the antiphospholipid syndrome. Lupus, 25(5), 451–462. https://doi.org/10.1177/0961203316636467
- Orlando Health. (n.d.). Warning Signs of Pulmonary Hypertension and How To Treat It. Orlando Health. Retrieved 2024, July 1, from: https://www.orlandohealth.com/content-hub/warning-signs-of-pulmonary-hypertension-and-how-to-treat-it
- Penn Medicine. (n.d.). Myelopathy. Penn Medicine. Retrieved 2024, July 1, from: https://www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/myelopathy
- de Godoy, J. M. P., Batigalia, F., & Braile, D. M. (2001). Superficial thrombophlebitis and anticardiolipin antibodies: report of association. Angiology, 52(2), 127. https://doi.org/10.1177/000331970105200206
- Polytarchou, K., Varvarousis, D., & Manolis, A. S. (2020). Cardiovascular Disease in Antiphospholipid Syndrome. Current Vascular Pharmacology, 18(6), 538–548. https://doi.org/10.2174/1570161117666190830101341
- Przysinda, A., Feng, W., & Li, G. (2020). Diversity of Organism-Wide and Organ-Specific Endothelial Cells. Current Cardiology Reports, 22(4), 19. https://doi.org/10.1007/s11886-020-1275-9
- Psych Scene Hub. (2024, April 26). Antiphospholipid Syndrome and the Brain (Neuropsychiatric Manifestations of APS) — Dr Sanil Rege. Psych Scene Hub. Retrieved from: https://psychscenehub.com/video/antiphospholipid-syndrome-aps-in-psychiatry-neuropsychiatric-manifestations-dr-sanil-rege/
- Psych Scene Hub. (2020, August 11). Neuropsychiatric Manifestations in Antiphospholipid Syndrome (APS) — Prof Graham Hughes. Psych Scene Hub. Retrieved from: https://psychscenehub.com/video/neuropsychiatric-manifestations-in-aps-by-prof-graham-hughes/
- Reyes, N., & Abe, K. (2023, May 1). Deep Vein Thrombosis & Pulmonary Embolism | CDC Yellow Book 2024. CDC. Retrieved from: https://wwwnc.cdc.gov/travel/yellowbook/2024/air-land-sea/deep-vein-thrombosis-and-pulmonary-embolism
- Ricarte, I. F., Dutra, L. A., Abrantes, F. F., Toso, F. F., Barsottini, O. G. P., Silva, G. S., de Souza, A. W. S., & Andrade, D. (2018). Neurologic manifestations of antiphospholipid syndrome. Lupus, 27(9), 1404–1414. https://doi.org/10.1177/0961203318776110
- Rodríguez-Olleros Rodríguez, C., & Díaz Curiel, M. (2019). Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. Journal of Osteoporosis, 2019, 2069176. https://doi.org/10.1155/2019/2069176
- Royal College of Psychiatrists. (n.d.). Neuropsychiatrist. Royal College of Psychiatrists. Retrieved 2024, July 1, from: https://www.rcpsych.ac.uk/become-a-psychiatrist/choose-psychiatry/what-is-psychiatry/types-of-psychiatrist/neuropsychiatry
- Sangle, S., D’Cruz, D. P., Khamashta, M. A., & Hughes, G. R. V. (2004). Antiphospholipid antibodies, systemic lupus erythematosus, and non-traumatic metatarsal fractures. Annals of the Rheumatic Diseases, 63(10), 1241–1243. https://doi.org/10.1136/ard.2003.016105
- Schmieder, S. J., & Krishnamurthy, K. (2023, July 4). Pyoderma Gangrenosum. In StatPearls. StatPearls Publishing. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK482223/
- Shi, M., Gao, W., Jin, Y., Zhu, J., Liu, Y., Wang, T., & Li, C. (2022). Antiphospholipid Syndrome-Related Pulmonary Embolism: Clinical Characteristics and Early Recognition. Frontiers in Cardiovascular Medicine, 9, 872523. https://doi.org/10.3389/fcvm.2022.872523
- Stanford Health Care. (n.d -a). Non-obstructive Coronary Artery Disease. Stanford Health Care. Retrieved 2024, July 1, from: https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/non-obstructive-coronary-artery-disease.html
- Stanford Health Care. (n.d -b). Endothelial Dysfunction. Stanford Health Care. Retrieved 2024, July 1, from: https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/endothelial-dysfunction.html
- State of Hawaii, Department of Health. (n.d.). Stroke Medical Terminology. State of Hawaii, Department of Health. Retrieved 2024, July 1, from: https://health.hawaii.gov/nt/stroke/stroke-medical-terminology/
- Suvajac, G., Stojanovich, L., & Milenkovich, S. (2007). Ocular manifestations in antiphospholipid syndrome. Autoimmunity Reviews, 6(6), 409–414. https://doi.org/10.1016/j.autrev.2006.11.005
- Takahashi, K., Ikeda, T., Yokoyama, K., & Kawakami, T. (2021). Cutaneous ulcer resembling pyoderma gangrenosum in a patient with antiphospholipid syndrome. Journal of Cutaneous Immunology & Allergy, 4(1). https://doi.org/10.1002/cia2.12143
- Tektonidou, M. G. (2022). Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. Journal of Autoimmunity, 128, 102813. https://doi.org/10.1016/j.jaut.2022.102813
- Tektonidou, M. G., & Moutsopoulos, H. M. (2006). Osteoarticular Manifestations of Antiphospholipid Syndrome. In M. A. Khamashta (Ed.), Hughes Syndrome: Antiphospholipid Syndrome (pp. 127–139). Springer. https://doi.org/10.1007/1-84628-009-5_12
- Uludag, G., Onghanseng, N., Tran, A. N. T., Hassan, M., Halim, M. S., Sepah, Y. J., Do, D. V., & Nguyen, Q. D. (2021). Current concepts in the diagnosis and management of antiphospholipid syndrome and ocular manifestations. Journal of Ophthalmic Inflammation and Infection, 11(1), 11. https://doi.org/10.1186/s12348-021-00240-8
- Velásquez, M., Rojas, M., Abrahams, V. M., Escudero, C., & Cadavid, Á. P. (2018). Mechanisms of Endothelial Dysfunction in Antiphospholipid Syndrome: Association With Clinical Manifestations. Frontiers in Physiology, 9, 1840. https://doi.org/10.3389/fphys.2018.01840
- Ward, T. (2024, April 5). What do long flights do to our bodies? National Geographic. Retrieved from: https://www.nationalgeographic.com/premium/article/long-flights-dehydration-pain-nausea-dvt
- Wei, M., Xu, Y., Xia, D., Li, J., & Dong, S. (2022). Care and Treatment for an Antiphospholipid Syndrome-Related Lower Limb Skin Ulcer Unhealed for 7 Years: A Case Report. The International Journal of Lower Extremity Wounds, 15347346221090079. https://doi.org/10.1177/15347346221090079
- World Health Organization. (2023, March 16). Hypertension. World Health Organization. Retrieved from: https://www.who.int/news-room/fact-sheets/detail/hypertension
- World Health Organization. (n.d.). Cardiovascular diseases. World Health Organization. Retrieved 2024, July 1, from: https://www.who.int/health-topics/cardiovascular-diseases
- Wu, K. K., & Thiagarajan, P. (1996). Role of endothelium in thrombosis and hemostasis. Annual Review of Medicine, 47, 315–331. https://doi.org/10.1146/annurev.med.47.1.315
- Xie, Z., Li, H., Qi, W., Li, J., Wu, C., Hu, C., Jiang, N., Wang, Q., Tian, X., Li, M., Zhao, J., Sui, R., & Zeng, X. (2022). Characteristics and risk factors of retinal vasculopathy in antiphospholipid syndrome. Lupus, 31(2), 178–186. https://doi.org/10.1177/09612033211069762
- Yelnik, C. M., Kozora, E., & Appenzeller, S. (2016). Non-stroke Central Neurologic Manifestations in Antiphospholipid Syndrome. Current Rheumatology Reports, 18(2), 11. https://doi.org/10.1007/s11926-016-0568-x
- Yu, R. Z. (n.d.). What Is APS “Brain Fog”? What Are Some Strategies to Help Manage It? Michigan Medicine. Retrieved 2024, July 1, from: https://medicine.umich.edu/dept/intmed/what-aps-%E2%80%9Cbrain-fog%E2%80%9D-what-are-some-strategies-help-manage-it
- Zhu, W., Wu, Y., Xu, M., Wang, J. Y., Meng, Y. F., Gu, Z., & Lu, J. (2015). Antiphospholipid antibody and risk of retinal vein occlusion: a systematic review and meta-analysis. Plos one, 10(4), e0122814. https://doi.org/10.1371/journal.pone.0122814

The Lowdown on Medications and Antiphospholipid Syndrome (Warfarin, Enoxaparin, DOACs, NSAIDs & More)
This article is part of the Antiphospholipid Syndrome (APS) resource library that I’m building up on my site. In this post, we will focus on medications and Antiphospholipid Syndrome. In particular, warfarin is a key medication for the management of APS, especially if you’ve experienced […]
Chronic voicesThis article is part of the Antiphospholipid Syndrome (APS) resource library that I’m building up on my site. In this post, we will focus on medications and Antiphospholipid Syndrome. In particular, warfarin is a key medication for the management of APS, especially if you’ve experienced blood clotting events in the past. We will also take a look at what DOACs (direct oral anticoagulants) are, how other medications such as NSAIDs (non steroidal anti-inflammatory drugs) can interact with warfarin, and exciting new drugs in the pipeline.
If there are specific terms or topics in this post that you were wondering about, such as injections, diet, bone health or something else, you can probably find in answers in the find the answers in the complete Antiphospholipid Syndrome A – Z guide here!
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Latest Research on Antiphospholipid Syndrome (2024 Edition)
- Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
- How Does Antiphospholipid Syndrome Affect The Body? (Beyond the Blood to Major Organs)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
Pin to Your Medications and Antiphospholipid Syndrome Boards:

*Disclaimer: This article is meant for educational purposes, and is based on my personal experiences as a patient. Whilst I have done my utmost to be meticulous in research, I am not a doctor, and nothing in this article should be substituted for medical advice. Please consult your own doctor before changing or adding any new treatment protocols. This post may also contain affiliate links. It will cost you nothing to click on them. I will get a small referral fee from purchases you make, which helps with the maintenance of this blog. Read our Privacy Policy page for more information. Thank you!
A Brief Introduction to Vitamin K
Vitamin K is important to know all about as a patient with Antiphospholipid Syndrome, as it interacts with warfarin and is a key contributor to the blood clotting process. It is a fat-soluble vitamin that comes in the form of vitamin K1 (phylloquinone) and vitamin K2 (a series of menaquinones). There is also a synthetic form – Vitamin K3 (menadione), which is no longer used in dietary supplements as they have been found to damage hepatic cells (Office of Dietary Supplements, 29 March, 2021).
Vitamin K1 is the main form of vitamin K in the human diet, and can mostly be found in green, leafy vegetables, certain fruits, and its absorption is increased in the presence of butter or oils. Vitamin K2 is mainly derived from fermented foods, dairy produce and animal-based sources.
According to Halder et al. (2019):
“VKDPs [vitamin K-dependent proteins] are categorized as hepatic and extra-hepatic VKDPs. Hepatic VKDPs include coagulation factors II, VII, IX, X, and anticoagulant protein C, protein S, and protein Z, all of which are involved in regulating blood coagulation. Extra-hepatic VKDPs include Matrix Gla protein (MGP), Osteocalcin, and Gla-rich protein (GRP). These VKDPs are primarily involved in maintaining bone homeostasis, as well as inhibiting ectopic calcification.”
In basic terms, what this means is that vitamin K, whether in K1 or K2 form, is an essential component of blood clotting processes, maintenance of bone health, and prevention of vessel mineralisation, depending on how and where they are metabolised. Whilst it’s important to be aware of vitamin K, I won’t deep dive into it in this post as I’d like to focus on medications and Antiphospholipid Syndrome.
-
Read Related Posts:
- Vitamin D & Vitamin K2: How They Boost Each Other in the Body
- The Causes & Dangers of Malabsorption & An Easy Way to Get Your Nutrients
Vitamin K Antagonists (VKAs)
Vitamin K antagonists (VKAs) are a class of medications used for the treatment and prevention of thrombosis. The most well-known and commonly used VKA is warfarin. For the sake of interest, other types of VKAs include: ethyl biscoumacetate, phenindione, anisindione, dicoumarol, phenprocoumon and diphenadione. There is a reason why warfarin is the most commonly used, as many of these other VKAs are erratic, highly toxic, or can cause other adverse side effects (Vardanyan and Hruby, 2006).
The administration of VKAs is convenient and practical, as they can be taken orally, and have a long half-life (warfarin has a half-life of 36 – 42 hours). Doctors are also able to adjust dosages for more precision in individual patients as needed, based on risk factors and medical history. VKAs work by antagonising the enzyme vitamin K epoxide reductase (VKOR) to prevent vitamin K from being recycled. This results in inhibition of the coagulation cascade, as many clotting factors rely on vitamin K to synthesise (Schein et al., 2016).
Coumarins
Vitamin K antagonists such as warfarin, acenocoumarol and phenprocoumon are derivatives of coumarin, which can be of natural or synthetic origin. In 1945, Karl Link experimented with 150 naturally occurring coumarin derivatives, before concluding that one compound was particularly active – warfarin. For a rare subset of patients with mutations in VKOR, VKAs are unfortunately low in efficacy or ineffective due to coumarin resistance (Kasperkiewicz et al., 2020). Read more about coumarins in the A to Z APS resource guide here.
Warfarin – THE Medication for Antiphospholipid Syndrome
Warfarin is a vitamin K antagonist, and is probably the most important drug for the management of Antiphospholipid Syndrome, and for the prevention of thrombosis. There are two brands of warfarin available – Coumadin and Marevan. It is important to stick to the same brand, as the formulation is not an exact match. What that means is that the anticoagulation effects can vary between each brand, and might mess your INR up. Do discuss with your doctor first, should you need to switch brands for any reason.
(Not so) fun fact: Warfarin also first started out as rat poison, but has declined in usage for such purposes due to an increase in resistance from these rodents, which is inheritable (Thijssen, 1995).
How Warfarin Works to Prevent Blood Clotting
Warfarin inhibits vitamin K dependent clotting factors II, VII, IX, and X, and also the anticoagulant proteins C and S (Crader et al., 1 May, 2023). This means that it inhibits multiple pathways in the blood clotting process, as compared to DOACs, which only disrupt a specific point. Specifically, prothrombin, FVII, FIX, protein C, and protein S are strictly related to blood coagulation processes (Girolami et al., 2018). Approximately 10% – 40% of factors II, VII, IX and X are inhibited by warfarin (Wadelius et al., 2004).
Vitamin K ‘activates’ these clotting factors via the enzyme, epoxide reductase, which is inhibited by warfarin. In rare cases, patients who are first taking warfarin may experience warfarin-induced skin necrosis. This is especially true for patients who are deficient in protein C, as it has the shortest half-life amongst them all. In such cases, patients are often co-administered heparin, as it has a quicker effect (Barmore et al., 24 February, 2023).
How Warfarin is Metabolised
Warfarin is a potent anticoagulant that is well absorbed by the body via the intestine with 90% bioavailability, and also offers high water solubility. It is then metabolised in the liver (hepatic), primarily through the CYP2C9 enzyme, with a half-life of approximately 20 – 60 hours. Other minor enzymatic pathways for metabolism include: CYP2C8, 2C18, 2C19, 1A2, and 3A4. How much of the drug gets metabolised is also dependent on each individual’s genetic variations (Patel et al., 24 March, 2023; Kasperkiewicz et al., 2020).
The warfarin molecule is further broken down into two forms – S- and R-warfarin, with S-warfarin 3 – 5 times more potent than R-warfarin. They are mainly broken down in the liver; metabolism of active S-warfarin is mainly via the CYP2C9 enzyme, and CYP enzymes CYP1A2 and CYP3A for R-warfarin.
Does Warfarin Interact with Other Medications?
The issue with warfarin is that it is also highly bound to serum albumin, which when combined with cytochrome P450 (CYP) metabolism, interacts with many drugs and foods. This results in warfarin either becoming more or less potent – thus increasing or decreasing its anticoagulatory effects, which can be risky either way for a patient with Antiphospholipid Syndrome (Tay et al., 2013).
Some drugs that interact with the CYP450 2C9 are cardiovascular medications such as amiodarone, and anti-infectives such as fluconazole. Some drugs that interact with CYP1A2 or CYP3A4 are quinolones and macrolides (bactericidal antibiotics). Inhibition of these enzymes can enhance the effects of warfarin, thus increasing the risk for bleeding further. Other drugs such as rifampicin (an antibiotic), carbamazepine (an anticonvulsant) and azathioprine (a DMARD) on the other hand, induces these enzymes and can decrease the potency of warfarin (Tay et al., 2013).
Highly Probable to Highly Improbable Interactions
In a systematic review by Holbrook et al. (2005), they categorise drugs that might potentially interact with warfarin from level 1 (highly probable) to level 4 (highly improbable). According to Holbrook et al. (2005):
“Of all 184 reviewed reports, 128 (70%) described a potentiation of warfarin’s effect, while inhibition and “no effect” reports each comprised 28 (15%). There were 34 reports of a major interaction—3 case reports of thrombosis associated with trazodone, sulfasalazine, and propofol and 31 case reports describing a major potentiation.”
Some drugs also yielded conflicting evidence for interaction with warfarin, i.e. terbinafine, ritonavir, and influenza vaccine. Linkins (2013) breaks down these medication categories by Holbrook et al. (2005) into a table according to type – antibiotics, antifungals, cardiovascular, cholesterol lowering agents, analgesics or antiinflammatory agents, and others. This is definitely not an exhaustive list of medications that interact with warfarin – there are way too many to be listed in one post, plus many others whose interactions are yet unknown.
What if I Have No Choice but to Take a Medication That Interacts with Warfarin?
Sometimes, taking medications that interact with warfarin is unavoidable, such as antibiotics for an infection, or an essential drug for another medical condition. For example, I was on carbamazepine and azathioprine for a period of time in the past, in a bid to control my epilepsy and Lupus (SLE) activity.
First, your rheumatologist (or doctor who manages your warfarin and Antiphospholipid Syndrome) should be consulted. They will then monitor and adjust your warfarin dose as needed, in combination with the other medications. As the medications I took were on a daily basis, that makes things easier as the main strategy to maintaining your target INR range is consistency in medication and food intake.
I eventually had to stop taking those medications not because they interacted with warfarin, but because they were ineffective or unsuitable for me for various reasons. Your doctor will also work with you to retitrate your warfarin dose when and if you need to stop taking these other medications. Remember that this applies to major dietary changes as well.
The main takeaway that I’d like to highlight is to always inform and work with your doctor when introducing a new medication that might potentially interfere with warfarin. Another important point is to always state that you’re on warfarin when you visit any new healthcare professional, including (especially) at the A&E/ER. In fact, you should have received a medical card that states that you are on warfarin – laminate this and keep it with you at all times so that you can show it to them.
-
Read Related Posts:
- Top Tips for Travelling with Chronic Illness & Disability (From a Girl Who Loves to Travel)
- My Recovery Time for Simultaneous Bilateral Patellar Tendon Rupture (With Lupus & Steroid Treatment)
- So This is What a Tonic Clonic Seizure Feels Like
- “But That’s Normal for Me” (Why I Mistook Dengue Fever for a Lupus Flare)
- A Car Accident & A Song Gone Silent (How Life Lessons from Chronic Illness Tide Me Through)
How to Check for Warfarin Interaction with Other Medications
Often, it can be a hassle and impractical to consult your doctor every time you need to take a new medication or food product. I personally use the MedScape app on my phone to do a quick check for medication interactions on the fly (I’ve seen doctors use it as well!). It tells you if there are minor or major contraindications, and why. It’s especially handy when I’m travelling. You can also tap into its comprehensive database to learn more about any other medication.
They also have a browser version here that you can use, plus other educational tools and resources on their website. Do note that it’s still important to cross-check with other verified sources and your doctor whenever possible, as not everything may be 100% accurate – that is impossible as research can sometimes be conflicting, and is also being constantly updated with new discoveries. (You can check out the latest research on Antiphospholipid Syndrome in this post.)
Screenshot Examples of the MedScape App:



How Much Warfarin Do I Need to Take with Antiphospholipid Syndrome?
The dose requirement of warfarin varies more than 10-fold amongst patients, due to genetic polymorphisms of CYP2C9, dietary differences, and other factors. According to Takahashi and Echizen (2003):
“Therapeutic targets measured by international normalized ratio (INR) of prothrombin time appear to differ between populations: INR of 2–3 for most indications in Caucasian patients1 and 1.5–2.5 for Asian patients.”
And according to Wadelius et al. (2004):
“CYP2C9 variants, age, weight, concurrent drug treatment and indication for treatment significantly influenced warfarin dosing in these patients, explaining 29% of the variation in dose. CYP3A5 did not affect warfarin dosing.”
Thus, there is no hard and fast rule as to how much warfarin one should take, as that needs to be titrated on an individual basis. Apart from the diverse genetic and dietary differences amongst patients, other factors need to be taken into account as well, such as comorbidities and the medications used to treat them, medical history, and other risk factors. If you’ve had had a thrombosis in the past, your INR target range might need to be higher as well, as that is an indication of an increased risk for blood clotting.
Pin to Your Warfarin, Medications and Antiphospholipid Syndrome Boards:

Tecarfarin – A Novel Vitamin K Antagonist in Phase III Trials
Tecarfarin is a novel VKA with a structural analog of warfarin, and can be measured via INR just like warfarin. It is being developed by Cardrenal Therapeutics for patients with implanted medical devices, end-stage renal disease (ESRD) and atrial fibrillation (AFib), although they are looking to expand its scope to include other patients who require anticoagulation too, such as in APS.
It has orphan drug with fast track designation from the FDA – meaning to say that it has governmental support for its development as a pharmaceutical agent for rare diseases. It is currently in Phase III clinical trials and in fact, since the time I drafted this section, it has already completed the ARIES-HM3 trial and presented its findings at the International Society for Heart & Lung Transplantation (ISHLT) 44th Annual Meeting & Scientific Sessions on June 3, 2024.
There are over hundreds of medications and foods that warfarin can interact with, due to the way it is metabolised by CYP2C9. Genetic variability in CYP2C9 gene can also affect the instability of INR. Tecarfarin on the other hand is metabolised by hCE-2, a pathway with no significant drug interactions, or genetic variability (Albrecht et al., 2017). It however, does not display any advantages over warfarin for people with VKORC1 polymorphisms.
In a multicentre study of 66 AFib patients for up to 12 weeks in a phase IIA trial, tecarfarin was shown to be well-tolerated without any adverse effects (Ellis et al., 2009). It was also shown to be well-tolerated in a small study of 40 healthy Chinese volunteers in a phase I trial (Zhou et al., 2023).
This means that tecarfarin might be a potential warfarin alternative or even replacement in future, as research has shown thus far similar effects as warfarin, minus the many interactions with other foods and drugs. I guess only time will tell, after it jumps through all the hoops of the essential clinical trials! (You can check out the latest research in this post.)
Enoxaparin – Another Important Medication in the APS Treatment Arsenal
We’ve covered quite a bit on warfarin, because it is so important when it comes to medications and Antiphospholipid Syndrome. Another important drug is enoxaparin (brand names: Lovenox and Clexane), which is also known as a “low molecular weight heparin” (LMWH). Two other FDA approved LMWHs in the USA are dalteparin (Fragmin) and tinzaparin (Innohep). Note that they should not be used interchangeably.
Enoxaparin is a medication that’s commonly substituted for warfarin, when APS patients need to pause their warfarin intake for whatever reason. It is often used as a bridge medication between surgical operations, where there is a higher chance of excessive bleeding.
This does not only include major surgeries, but also minor ones such as dental procedures, implantation of contraceptive devices, and procedures that require intramuscular injections such as the HPV vaccine. Enoxaparin has a quicker onset as compared to warfarin, and only has a half-life of about 4.5 – 7 hours (Cook, 2010). Hence, it is safer to be on enoxaparin during periods where excessive bleeding might be anticipated. You will need to do a warfarin reversal when switching to enoxaparin, which your doctor and gynaecologist will guide you through.
<!–You will need to do a warfarin reversal when switching to enoxaparin, which you can learn more about in the A – Z APS Resource Guide here.–>
Whilst derived from heparin, the final formulation of enoxaparin is different. It has a bioavailability of 90% when given in the subcutaneous form (injection into the fat just under the skin), and is a more stable and predictable drug that can be self-administered by patients at home (Jupalli and Iqbal, 28 August, 2023). Pregnant women with APS generally need to substitute warfarin for enoxaparin/LMWH during the term of their pregnancy, as warfarin can be harmful to foetuses. You can read more about pregnancy with Antiphospholipid Syndrome here.
How Does Enoxaparin / LMWH Work?
“[LMWHs and fondaparinux] target anti–factor Xa activity rather than AT [antithrombin]. With LMWH and fondaparinux, there is a reduced risk of heparin-induced thrombocytopenia (HIT), and monitoring of the aPTT is also not required, because the aPTT is insensitive to alterations in factor Xa.”
This means that the anticoagulatory mechanisms of LMWH differs from that of vitamin K antagonists, and that patients do not need to measure their INR like they do when on warfarin. I actually prefer being on enoxaparin as compared to warfarin despite the hassle of injections, because it gives me a chance to indulge in all the foods I love (yes, like broccoli and tofu…), as it does not interact with foods in the same way as VKAs do. Sadly, enoxaparin is not a good long-term medication as it is known to impair bone health and bone healing (Li et al., 2022). You can learn more about musculoskeletal manifestations in APS here.
Having said that, that does not mean that there are no drug interactions with enoxaparin. Mayo Clinic has listed some medications that can interact with enoxaparin here, and also certain medical conditions that should be highlighted to your doctor if you have them.
Differences Between ‘Standard’ Heparin and Low Molecular Weight Heparin
It is easy to get confused between enoxaparin/LMWH and heparin, since technically enoxaparin is derived from heparin. Many patients and sometimes even doctors refer to enoxaparin as heparin in casual speech. However, whilst enoxaparin can be self-administered by the patient via subcutaneous injections, unfractionated heparin (UFH) is usually given intravenously within a hospital setting, as there is a risk of Heparin Induced Thrombocytopenia (HIT).
HIT is a life-threatening condition where massive activation of platelets take place, with multi-cellular release of micro particles that contribute to hypercoagulability (Gruel et al., 2020). Compared to LMWH, unfractionated heparin is a highly variable drug as well, where almost 75% of patients fail to achieve the intended aPTT. Hence, patients need to be closely monitored when on unfractionated heparin (Krishnaswamy et al., 2010).
According to Krishnaswamy et al. (2010):
“Unfractionated heparin (UFH) exerts its effect by binding and inducing a conformational change in antithrombin (AT), converting AT to a more efficient inhibitor of circulating thrombin (factor IIa), factor Xa, factor IXa, factor XIIa, and kallikrein. Contributing to its efficiency, heparin can dissociate from the thrombin: AT complex and catalyze the activity of other AT molecules.”
Referring back to Lawrence’s (2011) explanation of the mechanisms of LMWHs, in comparison unfractionated heparin readily binds to antithrombin as well. This makes unfractionated heparin a more potent anticoagulant as compared to LMWH – but also brings with it more complications and bleeding risks. It is still an important medication to combat acute cases and medical emergencies however, such as pulmonary embolisms and heart attacks.
Pin to Your Medications and Antiphospholipid Syndrome Boards:

DOACs & Antiphospholipid Syndrome
DOACs stands for ‘Direct Oral Anticoagulants’, and they can be categorised into these main classifications: oral direct factor Xa inhibitors (apixaban, rivaroxaban, edoxaban, and betrixaban), and direct thrombin inhibitors (i.e. dabigatran) (Nasiri et al., 2022).
You can actually skip this entire section with a main takeaway – warfarin is still the mainstay drug for patients with Antiphospholipid Syndrome, especially for those who are high risk, or who have had a thrombosis before. However knowing me, I went down the research rabbit hole and found a lot of interesting information about these anticoagulants. I’ve actually asked my own rheumatologist before why I can’t be on DOACs as opposed to warfarin, as their benefits seemed much better. Now I know clearly why. If you’re interested to learn how DOACs work as an anticoagulant and why they aren’t quite recommended for APS patients, then read on!
What are DOACs and How do They Work?
DOACs are anticoagulants like VKAs, but their mechanism differs. The main advantages are that patients who are on DOACs need not monitor their diet or INR, and they have a rapid onset and offset of action (Pastori et al., 2021). One interesting finding about DOACs is that beyond their anticoagulation properties, they may also have an anti-inflammatory, anti-fibrotic and anti-angiogenic properties (Signorelli et al., 2018).
That might sound great, but APS patients are advised to use VKAs such as warfarin instead, especially if you’ve had a history of arterial thrombosis, or are triple positive, or even maybe double positive for antiphospholipid antibodies (Girón-Ortega and Girón-González, 2023; also see Bejjani et al., 2024). I personally have had multiple DVTs and a PE before, so my target INR with warfarin needs to be higher than the average APS patient, as I am at a greater risk of clotting. That can be better achieved via VKAs as compared to DOACs.
VKAs also target all phases of thrombin generation, whereas DOACs target only the initiation and/or propagation process. Rivaroxaban and apixaban in particular were found to be significantly inferior to VKAs for the prevention of recurrent thrombosis in APS patients (Girón-Ortega and Girón-González, 2023). DOACs also showed an increased risk of stroke amongst APS patients, and they may also be at a higher risk of thrombotic events (Shah et al., 2023).
Another problem with DOACs is that not all of them have an antidote at present, and more studies are yet to be done as to their safety and efficacy in actual patients. There are also specific groups of individuals whom DOACs are not suitable for, such as those with poor renal function, who have a prosthetic heart valve, a disorder of haemostasis, amongst others (Tran et al., 2014).
What is Thrombin?
A blood clot is also known as a thrombus, and thrombin plays an important role in the blood clotting process. Thrombin converts fibrinogen to fibrin, which is a tough protein that forms blood clots to seal wound sites. Thrombin is also the most potent platelet agonist (i.e. clots the blood). The more technical explanation, according to Posma et al. (2019):
“Activation of the blood coagulation cascade leads to fibrin deposition and platelet activation that are required for hemostasis. However, aberrant activation of coagulation can lead to thrombosis. Thrombi can cause tissue ischemia, and fibrin degradation products and activated platelets can enhance inflammation.”
DOACs: Direct Thrombin Inhibitors (DTIs)
According to Eriksson et al. (2011):
“DTIs directly neutralize thrombin by occupying the catalytic binding site, fibrinogen binding site, or both. DTIs also inhibit both fluid-phase and fibrin-bound thrombin.”
They do pretty much what their name states – inhibition of thrombin, which results in a reduction of blood clotting. A key advantage of DTIs is their ability to bind directly to thrombin, and they do not bind to other plasma proteins either (Lee and Ansell, 2011).
Dabigatran – The Only Approved DTI
The only approved DTI for use at present is dabigatran etexilate, which is a prodrug that metabolises into dabigatran in the body. Other DTIs such as ximelagatran have been withdrawn due to hepatotoxicity reports (Posma et al., 2019; van Ryn et al., 2013). Dabigatran is approved in over 70 countries, for the purpose of stroke prevention in patients with atrial fibrillation, and for the prevention of thrombosis after orthopaedic hip and knee surgery (van Ryn et al., 2013).
Whilst dabigatran seems to be a highly effective anticoagulant with a good safety profile thus far, it is important to note that it is primarily used in patients who don’t have Antiphospholipid Syndrome. International guidelines still indicate VKAs as the choice drug for patients with APS, especially if they have experienced a blood clotting event before, or are a high-risk patient, such as being triple positive (Pastori et al., 2021).
DOACs: Factor Xa Inhibitors
Factor Xa inhibitors on the other hand, reduces thrombin generation, and thus interferes with the process of fibrinogen to fibrin conversion. They also have no direct effect on platelet aggregation. It is theorised that this targeted action on factor Xa serves to limit the cascade of thrombin generation, and therefore less of the drug may be needed as compared to a DTI. Factor Xa also has minimal functions outside of coagulation, unlike thrombin, and side effects may be more contained (Cabral and Ansell, 2015).
Apixaban (Eliquis) – A Highly Selective Factor Xa Inhibitor
Apixaban (brand name: Eliquis) is a DOAC originally approved for atrial fibrillation (Afib) patients to reduce the risk of strokes and blood clots. It was later approved to treat DVTs and PEs (pulmonary embolisms) as well. It is a highly selective factor Xa inhibitor that exerts no effect on platelet aggregation, and mainly binds to plasma protein (Agrawal et al., 22 February, 2024). Side effects of taking apixaban are similar to other anticoagulant drugs, and include: bleeding, red or black, tarry stools, red, pink or brown urine, trouble breathing, dizziness, coughing up blood or material that looks like coffee grounds, and more.
Rivaroxaban (Xarelto) – Another Factor Xa Inhibitor
Rivaroxaban (brand name: Xarelto) is another type of DOAC under the factor Xa class. Based on the data available to date, rivaroxaban is less effective than VKAs in the prevention of recurrent thrombosis, but more research needs to be done as to its efficacy (Girón-Ortega and Girón-González, 2023).
A study of 120 high risk APS patients was terminated early due to the higher incidence of thromboembolic events for those who were on rivaroxaban, whereas none occurred in the group on warfarin (Pengo et al., 2018).
Pin to Your Medications and Antiphospholipid Syndrome Boards:

Current Reversal Agents Available for Anticoagulant Drugs
Patients who are on anticoagulants ironically run the risk of excessive bleeding or a haemorrhage, and not all DOACs have an antidote; the antidotes that are available are also expensive. In cases of severe bleeding, patients need to undergo reversal of their anticoagulant medication via a haemostatic agent.
According to Tomaselli et al. (2020) and Kustos and Fasinu (2019), these are the current reversal agents used for anticoagulant drugs during emergencies:
- Vitamin K Antagonists (warfarin) – Intravenous or oral vitamin K1. In addition, prothrombin complex concentrate (PCC) is preferred for immediate reversal, as compared to fresh frozen plasma (FFP) (Tran et al., 2013). Compared to FFP, 4F-PCCs contain approximately 25 times the concentration of vitamin K-dependent factors, and thus can be given at a smaller volume with a faster infusion rate.
- Vitamin K Antagonists (heparin) – Protamine.
- Indirect Thrombin Inhibitors (LMWH) – Protamine.
- DTI (dabigatran) – Idarucizumab (Praxbind). If unavailable, PCC or aPCC (activated prothrombin complex concentrate).
- Factor Xa Inhibitors (apixaban & rivaroxaban) – Andexanet Alfa. If unavailable, PCC or aPCC.
- Factor Xa (betrixaban & edoxaban) – Off-label treatment with high dose Andexanet Alfa. If unavailable, PCC or aPCC.
- In addition for the DOACs, activated charcoal may be considered for known recent ingestion in the last 2 – 4 hours.
This is just a brief, general guideline, and many more considerations need to be taken into account on an individual basis during an emergency. Please work with your own doctors and emergency care team for the best outcome.
A Bit More About Andexanet Alfa as a Reversal Agent
Andexanet alfa is administered via intravenous infusion with an onset time of 2 minutes. Side effects are primarily hot flashes and antibody development; they may also induce blood clotting ironically and result in strokes, DVTs, PEs, cardiac failure and more (Kustos and Fasinu, 2019; also see Escal et al., 2024). It is currently only approved for use as an antidote for apixaban and rivaroxaban, whilst its safety and efficacy is still being evaluated in relation to edoxaban and betrixaban.
Learn more about how APS affects major organs such as the lungs, brain and heart in this post.
Ciraparantag & Other Non-Specific Pharmacokinetic Antidotes to DOACs
Ciraparantag is a reversal agent still undergoing clinical trials, but shows promise as a non-immunogenic antidote for a wide array of anticoagulants (all types of DOACs, heparins and fondaparinux) (Escal et al., 2024).
Non-specific pharmacokinetic antidotes are sometimes used in addition to reversal agents to increase coagulation effects. According to Escal et al. (2024), these include:
- Activated Charcoal – Activated charcoal has the ability to absorb various substances in the body within the gastrointestinal tract. It may help to reduce blood concentrations of DOACs and thus its bioavailability. It also remains active for a long time and is low in cost.
- Haemodialysis – This is more for patients on DTIs (dabigatran), and especially if they have renal impairment. They are ineffective for Factor Xa inhibitors as they mainly bind to plasma proteins. It can be difficult to access during emergency situations however, as it requires trained healthcare professionals who know how to use specific equipment.
- Haemostatic Agents – These are useful when the type of DOAC the patient is on is unknown, or when standard reversal agents are unavailable. They include naPCC (non-activated prothrombin complex concentrates), FEIBA (factor eight inhibitor bypassing activity), rFVIIa (recombinant activated factor VII) and tranexamic acid. Note that these are second-line treatments and that more research still needs to be done to determine their exact effects and safety profile.
Pin to Your Medications and Antiphospholipid Syndrome Boards:

Proteases & Protease-Activated Receptor (PAR) Antagonists
Proteases are important regulators of cellular activity, and they communicate directly to cells via protease-activated receptors (PARs). There are four different PAR profiles, from PAR1 to PAR4, which are expressed widely in the body, from the brain to lungs, muscles, bones, bladder and more (Han et al., 2021). PAR1, PAR3 and PAR4 are activated by thrombin, whereas Factor Xa activates PAR2, although other proteases can also contribute to activation (Posma et al., 2019).
Cleavage of PARs
Inflammation is at the tail end of the blood coagulation cascade, as can be seen from a graphic in this paper by Posma et al. (2019).
According to Burzynski et al. (2019):
“The current principal link between coagulation and immunity is cleavage of PARs by thrombin, which produces cytokines and inflammation.”
This reveals a direct link between coagulation and the immune system, which is an exciting perspective. What this means is that future Antiphospholipid Syndrome treatments might shift to an immunological one, instead of merely targeting anticoagulation. Unfortunately, despite the implication of PARs in Antiphospholipid Syndrome, there are no ongoing trials for APS patients (Signorelli et al., 2018). Let’s keep our fingers crossed! (Read this post for the latest research on APS.)
Vorapaxar – An FDA-Approved PAR Antagonist
Vorapaxar is a first-in-class PAR1 antagonist approved for use by the FDA, for patients with coronary artery disease. Unfortunately, it is contraindicated for patients who have had thrombotic or bleeding events in the past. Like all antiplatelet agents, vorapaxar increases the risk of bleeding, which can be fatal. It also has a long half-life and no antidote (Han et al., 2021; Signorelli et al., 2018).
Another PAR-1 antagonist is atopaxar, but research has been discontinued as clinical trials in phase II demonstrated a high risk for bleeding with the drug (Zhao et al., 2013).
NSAIDs & Antiphospholipid Syndrome
NSAIDs (Non-Steroidal Anti-Inflammatory Drugs) are a class of anti-inflammatories that people often take for generic pain relief. You’re probably familiar with these over-the-counter drugs that go by the names of: Ibuprofen, Naproxen and Aspirin. Brand names include Neurofen, Advil, Motrin, Aleve, Alka-Seltzer, Pepto-Bismol, and more.
To be honest, NSAIDS would probably work better for my Lupus and Sjögren’s pains because of their anti-inflammatory properties, but I can’t take them due to interactions with warfarin. As I’m also allergic to paracetamol (Panadol), I can only rely on opioid classes of painkillers for pain relief.
NSAIDs interfere with blood clotting by inhibiting platelet function. Platelets (also known as thrombocytes) are essential components in our blood that help with clotting. Hence, patients who are on blood thinning medications have a higher risk of bleeding with NSAIDs.
There is also an increased risk of gastrointestinal bleeding and peptic ulcers with NSAIDs due to the mechanisms of the medication (Drini, 2017), which is also the biggest concern my rheumatologist has with them. I only use NSAIDs when absolutely necessary, such as during a high fever, and inform my healthcare team when I do so. I also take extra measures to protect my stomach such as eating proper meals, and check my own INR regularly.
-
Read Related Posts:
- Why Painkillers are One of My Biggest Allies for a Decent Quality of Life
- Oral Spray Vitamins: A Quick & Easy Way to Get Your Nutrients with Chronic Illness
- What’s it Like to be on a High Dose of Steroids? (And the First Question You Will Definitely Ask)
- Drink Pure Wine Review (A Product That Excites Me as a Person with Chronic Illness)
- 12 Visible Evidence of a Body Gone Rogue (Is Invisible Illness Truly Invisible?)
Pin to Your Medications and Antiphospholipid Syndrome Boards:

Conclusion to Medications and Antiphospholipid Syndrome
In this post, we have covered medications and Antiphospholipid Syndrome, as well as some of the important drugs that can interact with warfarin, and new medications that are undergoing clinical trials.
I hope that this post on medications and Antiphospholipid Syndrome has been insightful and useful to you whether as an APS patient, or someone who is caring for one. Should you have any questions, experiences to share, or corrections (I am not a doctor, after all!), feel free to leave a comment below. Don’t forget to check out the rest of the posts in the Antiphospholipid Syndrome series listed below as well. Wishing you all the best that life can still bring!
P.s. If you’d like to support the work I do, you can keep me fueled with coffee using the button below. You can also sign up for our mailing list so you don’t miss out on our latest posts. You will receive an e-book full of uplifting messages, quotes and illustrations, as a token of appreciation!
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Antiphospholipid Syndrome Diagnosis: The A to Z Guide as a Patient
- Latest Research on Antiphospholipid Syndrome (2024 Edition)
- Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
- How Does Antiphospholipid Syndrome Affect The Body? (Beyond the Blood to Major Organs)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- http://What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
Pin to Your Medications and Antiphospholipid Syndrome Boards:

- Agrawal, A., Kerndt, C. C., & Manna, B. (22 February, 2024). Apixaban. In StatPearls. StatPearls Publishing. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK507910/
- Albrecht, D., Ellis, D., Canafax, D. M., Combs, D., Druzgala, P., Milner, P. G., & Midei, M. G. (2017). Pharmacokinetics and pharmacodynamics of tecarfarin, a novel vitamin K antagonist oral anticoagulant. Thrombosis and Haemostasis, 117(04), 706-717. https://doi.org/10.1160/TH16-08-0623
- Barmore, W., Bajwa, T., & Burns, B. (24 February, 2023). Biochemistry, Clotting Factors. In StatPearls. StatPearls Publishing. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK507850/
- Bejjani, A., Khairani, C. D., Assi, A., Piazza, G., Sadeghipour, P., Talasaz, A. H., Fanikos, J., Connors, J. M., Siegal, D. M., Barnes, G. D., Martin, K. A., Angiolillo, D. J., Kleindorfer, D., Monreal, M., Jimenez, D., Middeldorp, S., Elkind, M. S. V., Ruff, C. T., Goldhaber, S. Z., … Bikdeli, B. (2024). When Direct Oral Anticoagulants Should Not Be Standard Treatment. Journal of the American College of Cardiology, 83(3), 444–465. https://doi.org/10.1016/j.jacc.2023.10.038
- Burzynski, L. C., Humphry, M., Pyrillou, K., Wiggins, K. A., Chan, J. N. E., Figg, N., Kitt, L. L., Summers, C., Tatham, K. C., Martin, P. B., Bennett, M. R., & Clarke, M. C. H. (2019). The Coagulation and Immune Systems Are Directly Linked through the Activation of Interleukin-1α by Thrombin. Immunity, 50(4), 1033-1042.e6. https://doi.org/10.1016/j.immuni.2019.03.003
- Cabral, K. P., & Ansell, J. E. (2015). The role of factor Xa inhibitors in venous thromboembolism treatment. Vascular Health and Risk Management, 11, 117–123. https://doi.org/10.2147/VHRM.S39726
- Cadrenal Therapeutics, Inc. (3 June, 2024). Cadrenal Therapeutics Highlights Presentation Of New Trial Data at Ishlt Conference Demonstrating The Importance Of Anticoagulation Quality In LVAD Patients. Cadrenal Therapeutics, Inc. Retrieved from: https://www.cadrenal.com/investors/press-releases/detail/cadrenal-therapeutics-provides-third-quarter-2023-corporate-update
- Cadrenal Therapeutics, Inc. (9 November, 2023). Cadrenal Therapeutics Provides Third Quarter 2023 Corporate Update. U.S. Securities And Exchange Commission. Retrieved from: https://www.sec.gov/Archives/edgar/data/1937993/000121390023084969/ea187953ex99-1_cadrenal.htm
- Cadrenal Therapeutics, Inc. (n.d.). Tecarfarin. A late-stage novel therapy with orphan drug and Fast Track designations. Cadrenal Therapeutics, Inc. Retrieved from: https://www.cadrenal.com/tecarfarin/
- Cleveland Clinic. (28 April, 2022). Platelets. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/body/22879-platelets
- Cook, B. W. (2010). Anticoagulation Management. Seminars in Interventional Radiology, 27(4), 360–367. https://doi.org/10.1055/s-0030-1267849
- Crader, M. F., Johns, T., & Arnold, J. K. (1 May, 2023). Warfarin Drug Interactions. In StatPearls. StatPearls Publishing. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK441964/
- Department of Health, Government of Western Australia. (n.d.). Warfarin. HealthyWA. Retrieved from: https://www.healthywa.gov.au/Articles/A-Z/Warfarin
- Drini, M. (2017). Peptic ulcer disease and non-steroidal anti-inflammatory drugs. Australian Prescriber, 40(3), 91–93. https://doi.org/10.18773/austprescr.2017.037
- Drugs .com. (n.d.). Enoxaparin (Ingredient). Drugs .com. Retrieved from: https://www.drugs.com/ingredient/enoxaparin.html
- Ellis, D. J., Usman, M. H., Milner, P. G., Canafax, D. M., & Ezekowitz, M. D. (2009). The First Evaluation of a Novel Vitamin K Antagonist, Tecarfarin (ATI-5923), in Patients With Atrial Fibrillation. Circulation, 120(12), 1029–1035. https://doi.org/10.1161/CIRCULATIONAHA.109.856120
- Eriksson, B. I., Quinlan, D. J., & Eikelboom, J. W. (2011). Novel Oral Factor Xa and Thrombin Inhibitors in the Management of Thromboembolism. Annual Review of Medicine, 62(Volume 62, 2011), 41–57. https://doi.org/10.1146/annurev-med-062209-095159
- Escal, J., Lanoiselée, J., Poenou, G., Zufferey, P., Laporte, S., Mismetti, P., & Delavenne, X. (2024). Latest advances in the reversal strategies for direct oral anticoagulants. Fundamental & Clinical Pharmacology. https://doi.org/10.1111/fcp.12992
- Girolami, A., Ferrari, S., Cosi, E., Santarossa, C., & Randi, M. L. (2018). Vitamin K-Dependent Coagulation Factors That May be Responsible for Both Bleeding and Thrombosis (FII, FVII, and FIX). Clinical and Applied Thrombosis/Hemostasis, 24(9 Suppl), 42S-47S. https://doi.org/10.1177/1076029618811109
- Girón-Ortega, J. A., & Girón-González, J. A. (2023). Direct-acting oral anticoagulants in antiphospholipid syndrome: A systematic review. Medicina Clínica (English Edition), 161(2), 65–77. https://doi.org/10.1016/j.medcle.2023.03.017
- Gruel, Y., De Maistre, E., Pouplard, C., Mullier, F., Susen, S., Roullet, S., Blais, N., Le Gal, G., Vincentelli, A., Lasne, D., Lecompte, T., Albaladejo, P., Godier, A., Albaladejo, P., Belisle, S., Blais, N., Bonhomme, F., Borel-Derlon, A., Borg, J. Y., … Zufferey, P. (2020). Diagnosis and management of heparin-induced thrombocytopenia. Anaesthesia Critical Care & Pain Medicine, 39(2), 291–310. https://doi.org/10.1016/j.accpm.2020.03.012
- Halder, M., Petsophonsakul, P., Akbulut, A. C., Pavlic, A., Bohan, F., Anderson, E., Maresz, K., Kramann, R., & Schurgers, L. (2019). Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. International Journal of Molecular Sciences, 20(4), Article 4. https://doi.org/10.3390/ijms20040896
- Han, X., Nieman, M. T., & Kerlin, B. A. (2020). Protease‐activated receptors: An illustrated review. Research and Practice in Thrombosis and Haemostasis, 5(1), 17–26. https://doi.org/10.1002/rth2.12454
- Harvard Medical School. (16 December, 2019). Bad mix: Blood thinners and NSAIDs. Harvard Health Publishing. Retrieved from: https://www.health.harvard.edu/diseases-and-conditions/bad-mix-blood-thinners-and-nsaids
- Holbrook, A. M., Pereira, J. A., Labiris, R., McDonald, H., Douketis, J. D., Crowther, M., & Wells, P. S. (2005). Systematic Overview of Warfarin and Its Drug and Food Interactions. Archives of Internal Medicine, 165(10), 1095–1106. https://doi.org/10.1001/archinte.165.10.1095
- Jupalli, A., & Iqbal, A. M. (28 August, 2023). Enoxaparin. In StatPearls. StatPearls Publishing. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK539865/
- Kasperkiewicz, K., Ponczek, M. B., Owczarek, J., Guga, P., & Budzisz, E. (2020). Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules, 25(6), 1465. https://doi.org/10.3390/molecules25061465
- Krishnaswamy, A., Lincoff, A. M., & Cannon, C. P. (2010). The Use and Limitations of Unfractionated Heparin. Critical Pathways in Cardiology, 9(1), 35. https://doi.org/10.1097/HPC.0b013e3181d29713
- Kustos, S. A., & Fasinu, P. S. (2019). Direct-Acting Oral Anticoagulants and Their Reversal Agents-An Update. Medicines (Basel, Switzerland), 6(4), 103. https://doi.org/10.3390/medicines6040103
- Lawrence, P. F. (2011). Chapter 78—Pharmacologic Adjuncts to Endovascular Procedures. In W. S. Moore & S. S. Ahn (Eds.), Endovascular Surgery (Fourth Edition) (pp. 807–813). W.B. Saunders. https://doi.org/10.1016/B978-1-4160-6208-0.10078-3
- Lee, C. J., & Ansell, J. E. (2011). Direct thrombin inhibitors. British Journal of Clinical Pharmacology, 72(4), 581–592. https://doi.org/10.1111/j.1365-2125.2011.03916.x
- Li, Y., Liu, L., Li, S., Sun, H., Zhang, Y., Duan, Z., & Wang, D. (2022). Impaired bone healing by enoxaparin via inhibiting the differentiation of bone marrow mesenchymal stem cells towards osteoblasts. Journal of Bone and Mineral Metabolism, 40(1), 9–19. https://doi.org/10.1007/s00774-021-01268-5
- Mayo Clinic. (1 February, 2024). Enoxaparin (Intravenous Route, Subcutaneous Route, Injection Route). Mayo Clinic. Retrieved from: https://www.mayoclinic.org/drugs-supplements/enoxaparin-intravenous-route-subcutaneous-route-injection-route/side-effects/drg-20063670?p=1
- MedlinePlus. (15 April, 2023). Rivaroxaban. Bethesda (MD): National Library of Medicine (US). Retrieved from: https://medlineplus.gov/druginfo/meds/a611049.html
- MedlinePlus. (15 November, 2023). Apixaban. Bethesda (MD): National Library of Medicine (US). Retrieved from: https://medlineplus.gov/druginfo/meds/a613032.html
- Nasiri, A., AlQahtani, A., Rayes, N. H., AlQahtani, R., Alkharras, R., & Alghethber, H. (2022). Direct oral anticoagulant: Review article. Journal of Family Medicine and Primary Care, 11(8), 4180–4183. https://doi.org/10.4103/jfmpc.jfmpc_2253_21
- Office of Dietary Supplements. (29 March, 2021). Vitamin K Fact Sheet for Health Professionals. National Institutes of Health. Retrieved from: https://ods.od.nih.gov/factsheets/VitaminK-HealthProfessional/
- Pastori, D., Menichelli, D., Cammisotto, V., & Pignatelli, P. (2021). Use of Direct Oral Anticoagulants in Patients With Antiphospholipid Syndrome: A Systematic Review and Comparison of the International Guidelines. Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.715878
- Pengo, V., Denas, G., Zoppellaro, G., Jose, S. P., Hoxha, A., Ruffatti, A., Andreoli, L., Tincani, A., Cenci, C., Prisco, D., Fierro, T., Gresele, P., Cafolla, A., De Micheli, V., Ghirarduzzi, A., Tosetto, A., Falanga, A., Martinelli, I., Testa, S., … Banzato, A. (2018). Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood, 132(13), 1365–1371. https://doi.org/10.1182/blood-2018-04-848333
- Patel, S., Singh, R., Preuss, C. V., & Patel, N. (24 March, 2023). Warfarin. In StatPearls. StatPearls Publishing. Retrieved from: http://www.ncbi.nlm.nih.gov/books/NBK470313/
- Posma, J. J., Grover, S. P., Hisada, Y., Owens, A. P., Antoniak, S., Spronk, H. M., & Mackman, N. (2019). Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(1), 13–24. https://doi.org/10.1161/ATVBAHA.118.311655
- Ruff, C. T. (17 May, 2022). Direct Oral Anticoagulants (DOACs) vs. Warfarin: Key Differences. North American Thrombosis Forum [NATF]. Retrieved from: https://thrombosis.org/2020/11/doacs-vs-warfarin/
- Schein, J. R., White, C. M., Nelson, W. W., Kluger, J., Mearns, E. S., & Coleman, C. I. (2016). Vitamin K antagonist use: Evidence of the difficulty of achieving and maintaining target INR range and subsequent consequences. Thrombosis Journal, 14(1), 14. https://doi.org/10.1186/s12959-016-0088-y
- Shah, B. B., Shankar, A., Kumar, V., Kumar, S., Malik, U. A., Majeed, A., … & Ahmed, S. (2023). Direct oral anticoagulants vs. vitamin K antagonists in patients with antiphospholipid syndrome: a systematic review and meta-analysis. Annals of Medicine and Surgery, 85(7), 3574-3582. https://doi.org/10.1097/MS9.0000000000000903
- Signorelli, F., Balbi, G. G. M., Domingues, V., & Levy, R. A. (2018). New and upcoming treatments in antiphospholipid syndrome: A comprehensive review. Pharmacological Research, 133, 108–120. https://doi.org/10.1016/j.phrs.2018.04.012
- Takahashi, H., & Echizen, H. (2003). Pharmacogenetics of CYP2C9 and interindividual variability in anticoagulant response to warfarin. The Pharmacogenomics Journal, 3(4), 202–214. https://doi.org/10.1038/sj.tpj.6500182
- Tay, K. H., Shantsila, E., & Lip, G. Y. H. (2013). The Coagulation Pathway and Approaches to Anticoagulation. In G. Y. Lip & E. Shantsila (Eds.), Handbook of Oral Anticoagulation (pp. 1–5). Springer Healthcare Ltd. https://doi.org/10.1007/978-1-908517-96-8_1
- Thijssen, H. H. W. (1995). Warfarin-based rodenticides: Mode of action and mechanism of resistance. Pesticide Science, 43(1), 73–78. https://doi.org/10.1002/ps.2780430112
- Tomaselli, G. F., Mahaffey, K. W., Cuker, A., Dobesh, P. P., Doherty, J. U., Eikelboom, J. W., Florido, R., Gluckman, T. J., Hucker, W. J., Mehran, R., Mess, é S. R., Perino, A. C., Rodriguez, F., Sarode, R., Siegal, D. M., & Wiggins, B. S. (2020). 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants. Journal of the American College of Cardiology, 76(5), 594–622. https://doi.org/10.1016/j.jacc.2020.04.053
- Tran, H., Joseph, J., Young, L., McRae, S., Curnow, J., Nandurkar, H., Wood, P., & McLintock, C. (2014). New oral anticoagulants: A practical guide on prescription, laboratory testing and peri-procedural/bleeding management. Internal Medicine Journal, 44(6), 525–536. https://doi.org/10.1111/imj.12448
- Tran, H. A., Chunilal, S. D., Harper, P. L., Tran, H., Wood, E. M., & Gallus, A. S. (2013). An update of consensus guidelines for warfarin reversal. Medical Journal of Australia, 198(4), 198–199. https://doi.org/10.5694/mja12.10614
- U.S. Food & Drug Administration. (2 June, 2018). Generic Enoxaparin Questions and Answers. FDA. Retrieved from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/generic-enoxaparin-questions-and-answers
- van Ryn, J., Goss, A., Hauel, N., Wienen, W., Priepke, H., Nar, H., & Clemens, A. (2013). The Discovery of Dabigatran Etexilate. Frontiers in Pharmacology, 4, 12. https://doi.org/10.3389/fphar.2013.00012
- Vardanyan, R. S., & Hruby, V. J. (2006). 24—Anticoagulants, Antiaggregants, Thrombolytics, and Hemostatics. In R. S. Vardanyan & V. J. Hruby (Eds.), Synthesis of Essential Drugs (pp. 323–335). Elsevier. https://doi.org/10.1016/B978-044452166-8/50024-8
- Wadelius, M., Sörlin, K., Wallerman, O., Karlsson, J., Yue, Q.-Y., Magnusson, P. K. E., Wadelius, C., & Melhus, H. (2004). Warfarin sensitivity related to CYP2C9, CYP3A5, ABCB1 (MDR1) and other factors. The Pharmacogenomics Journal, 4(1), 40–48. https://doi.org/10.1038/sj.tpj.6500220
- Zhao, H.-P., Jiang, H.-M., & Xiang, B.-R. (2013). Discontinued drugs in 2012: Cardiovascular drugs. Expert Opinion on Investigational Drugs, 22(11), 1437–1451. https://doi.org/10.1517/13543784.2013.832198
- Zhou, Q., Wang, Z., Wang, H., Chen, Z., Li, X., Dai, X., Zhang, Y., Yu, X., Zhou, R., & Hu, W. (2023). Safety and Tolerability of Tecarfarin (ATI-5923) in Healthy Chinese Volunteers: Multiple Oral Dose-Escalation Phase I Trial. American Journal of Cardiovascular Drugs, 23(1), 101–112. https://doi.org/10.1007/s40256-022-00562-5
References:

Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
This post is part of the Antiphospholipid Syndrome (APS) resource library that I’m building up on my site for patients, as a patient who’s lived with it for more than two decades myself. This article in particular will focus on all things related to the […]
Chronic voicesThis post is part of the Antiphospholipid Syndrome (APS) resource library that I’m building up on my site for patients, as a patient who’s lived with it for more than two decades myself. This article in particular will focus on all things related to the female sex and women’s health in Antiphospholipid Syndrome. It will cover topics such as pregnancy, miscarriage, menstruation, menopause, risk factors, birth control and other general knowledge.
I have taken the time to research relevant medical journals, and also share my personal experiences where appropriate. I hope you find it useful, whether as a patient or as a supporter. If there are specific terms used in this post that you were wondering about, such as antiphospholipid antibodies (aPLs), correlations with other autoimmune diseases such as Lupus, the reversal protocol and more, then I’d recommend that you check out the complete A – Z guide plus other resources in the series below.
Antiphospholipid Syndrome Diagnosis: The A – Z Guide as a Patient
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Latest Research on Antiphospholipid Syndrome (2024 Edition)
- The Lowdown on Medications & Antiphospholipid Syndrome (Warfarin, Enoxaparin, DOACs, NSAIDs & More)
- How Does Antiphospholipid Syndrome Affect The Body? (Beyond the Blood to Major Organs)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
*Disclaimer: This article is meant for educational purposes, and is based on my personal experiences as a patient. Whilst I have done my utmost to be meticulous in research, I am not a doctor, and nothing in this article should be substituted for medical advice. Please consult your own doctor before changing or adding any new treatment protocols. This post may also contain affiliate links. It will cost you nothing to click on them. I will get a small referral fee from purchases you make, which helps with the maintenance of this blog. Read our Privacy Policy page for more information. Thank you!
Pin to Your Women’s Health in Antiphospholipid Syndrome Boards:

Women’s Health in Antiphospholipid Syndrome
The terms for ‘women’ and ‘female’ used in this article will mostly be referring to those who were assigned female at birth, and will take into account genetic differences and their links with autoimmunity. Females typically have two X chromosomes, whereas males have an X and Y chromosome. Females account for around 80% of all autoimmune disease cases as well, and recent studies have shown that specific X-linked genes may be contributors to autoimmune disease. According to Dou et al. (2024):
“To make the gene expression output roughly equivalent between females and males, every cell in a female’s body epigenetically silences one of two X chromosomes via the action of the long non-coding RNA (lncRNA) Xist. Xist is an ∼17-kb lncRNA (19 kb in human) that is transcribed only from the inactive X chromosome and thus not expressed in males.”
Whilst exactly how, when and where is still sketchy, there is no doubt that there can be further Antiphospholipid Syndrome related complications simply by being female. After all, APS is a blood clotting autoimmune disorder, and as women we get periods every month on average, and also need to go through the pregnancy process if we so choose to – all of which involves the immune system and blood.
Epidemiology of APS – Males vs Females
Antiphospholipid Syndrome is more commonly found in women than in men, with a ratio of about 3.5:1. It is also more often secondary APS (SAPS) in women, whereas men are more prone to primary APS (PAPS). Women present with strokes, livedo and headaches more frequently than in men. Whereas male PAPS patients present more with myocardial infarction, mesenteric and hepatic vein thrombosis (Kaul and Hughes, 2023).
Other studies have also shown greater cerebrovascular disease in women, whilst men had gastrointestinal involvement more frequently (Jara et al., 2005). This is also an interesting table which shows the comorbidities associated with APS and antiphospholipid antibodies (aPLs) positivity in the general population, from a compilation of various research papers (p.s. link sometimes needs to be clicked twice to open for some unknown reason). It includes the various aPLs found in women in different types of pregnancy incidents, ages when women first experienced a stroke, patients with comorbidities, and more (Dabit et al., 2021).
Read this post for more information on how Antiphospholipid Syndrome can affect the entire body.
Pin to Your Women’s Health in Antiphospholipid Syndrome Boards:

Menstruation & Antiphospholipid Syndrome
It is not unusual for us ladies to experience heavier periods whilst taking anticoagulants, or to see some blood clots discharged. Something important to know about are ovarian cysts. Most of these cysts are common and harmless, and occur even in healthy women. They usually get reabsorbed into the body or are passed out, but sometimes they can rupture.
When this happens, it is called an Ovarian Cyst Rupture, and I can tell you from two first-hand experiences that it is not pleasant. I nearly died both times. This was also the reason why my healthcare team decided to put me on birth control (Nexplanon), to prevent further episodes. A benefit of being on birth control, at least for me, is that I have experienced lighter periods with fewer period cramps since then.
The Relation Between Oestrogen & APS
Or estrogen, depending on where you come from! Oestrogen is a steroid hormone associated with menstruation (Delgado and Lopez-Ojeda, 2023). There are a few different types – estrone (E1), estradiol (E2), estriol (E3), and etestrol (E4) – and they collectively regulate the development and function of the female reproductive system. Males also produce oestrogen, primarily in the testis (Harding and Heaton, 2022).
Beyond its role as a sex hormone, oestrogens and their receptors also play a role in modulating innate immune responses against infections, from viral to bacterial, parasitic and fungal. They display anti-inflammatory effects and also help the body with wound healing and repair (Harding and Heaton, 2022). A powerful hormone, no doubt!
However, oestrogen also plays a role in pain flares for rheumatoid autoimmune disorders, such as SLE (Lupus) and Rheumatoid Arthritis. Oestrogens also increase the risk of both arterial and venous thrombosis, and have effects on almost every cell in the body. The technical explanation, according to Abou-Ismail et al. (2020):
“Estrogen leads to increased thrombin generation and fibrin clot formation by increasing the levels of variable coagulation proteins and decreasing the levels of anticoagulant proteins.”
And according to Manukyan et al. (2020):
“Our findings show the ability of E2 [high-17β-estradiol] to promote proinflammatory and procoagulatory phenotype of innate immune cells in individuals with aPL [antiphospholipid antibodies] positivity. Our data highlights the significant impact of female hormones on the activation of immune cells in the presence of aPL.”
Oestrogen also increases the risk of blood clots when used in contraceptives or as postmenopausal hormone replacement therapy. This can also happen in men who use them as treatment for coronary disease, or in sex-change treatment (Rosendaal et al., 2002). Thus, it is best to avoid treatments and forms of contraception that contain oestrogen if you have Antiphospholipid Syndrome.
Ovarian Cyst Ruptures in Women with Antiphospholipid Syndrome
Ovarian cyst ruptures are extremely painful and can be life-threatening. For women with Antiphospholipid Syndrome who are on some form of anticoagulation treatment, the blood thinning effects of the medication(s) can compound the problem. Let’s take a closer look at how and why they happen.
Types of Functional Cysts
There are two types of functional cysts that your ovaries grow every month – a follicle cyst and a corpus luteum cyst. A follicular cyst occurs when the follicle doesn’t rupture during ovulation. A corpus luteum cyst occurs after the egg is released, and the opening becomes blocked in the corpus luteum. Most functional ovarian cysts are harmless and resolve on their own.
However, an ovarian cyst rupture can occur sometimes during menstruation. For a healthy female, usually this gets passed out as a blood clot, or reabsorbed by the body, with few to no incidences. If there are symptoms, they may include sudden abdominal or pelvic pain, pain with fever and vomiting, or signs of shock and weakness.
Hemoperitoneum in Females with APS
For a female with Antiphospholipid Syndrome, such ruptures can sometimes cause internal bleeding, no thanks to their blood thinning medications. This is also known as a hemoperitoneum, where excess blood accumulates in the abdominal or pelvic cavity, and it is a medical emergency.
This doesn’t happen every period of course, but I’ve been unlucky to have had two ovarian cyst rupture episodes that were of life-threatening status. The pain was acute and came on suddenly. Within 4 hours I was doubled over, swollen and bloated with pain, and had to crawl to the hospital.
They will usually do an abdominal ultrasound at the A&E/ER to check for free fluid. Emergency surgery is usually required, but generally avoided for patients who are on anticoagulants, until the blood thinning effects of their medications have been counteracted.
The second time I had an ovarian cyst rupture, the nurses at the A&E were rather junior, so I had to insist on the ultrasound. The senior doctor later confirmed the diagnosis, and I was immediately placed in the high emergency section, given strong painkillers, and injected with three different types of blood clotting agents. RBC (red blood cell) counts may fall, but this can be due to dehydration as well.
-
Read Related Posts:
- 12 Visible Evidence of a Body Gone Rogue (Is Invisible Illness Truly Invisible?)
- Why Painkillers are One of My Biggest Allies for a Decent Quality of Life
- YuYu Bottle Review: Hot Water Bottle for ‘Surround Warmth’ Pain Relief
- What It Feels Like to be Suddenly Disabled
- “But That’s Normal for Me” (Why I Mistook Dengue Fever for a Lupus Flare)
Birth Control as Prevention for Bleeding Risk in Women with Antiphospholipid Syndrome
As a result of these ovarian cyst rupture episodes, my gynaecologist suggested birth control to prevent ovulation, which would reduce the chances of a reoccurrence.
There are a few types of birth controls, and they contain the hormones oestrogen, or progestin, or both. As mentioned previously, oestrogen increases the risk of blood clots. Studies have also demonstrated that progestins in combination oral contraceptives also play a role to a lesser degree (Rosendaal, 2002). You can view a table of contraceptive recommendations for women with APS here (Sammaritano, 2021).
As I have many comorbidities such as Lupus and Sjögren’s disease and am severely immunocompromised, my gynaecologist did not recommend intrauterine devices (IUDs) either due to the higher chance for infections. I personally use Nexplanon, as suggested by my own gynaecologist.
Etonogestrel (Implanon & Nexplanon) – What I Use for Birth Control as a Female with APS
Etonogestrel is a progestin hormone under the brand names of Implanon and Nexplanon. It comes in the form of a small implant, and your gynaecologist will make a small cut in your arm to insert it subdermally. You will most likely need to do a reversal of your blood thinning medication beforehand, just in case of excessive bleeding. Your rheumatologist/doctor and gynaecologist will work closely with you to do the reversal protocol, which you can read more about here.
What I like about the etonogestrel implant is that once it’s inserted, it lasts for 3 years and you can go about your life as per usual. And should you wish to try for pregnancy, you can simply remove it and start trying pretty much immediately.
Pin to Your Women’s Health in Antiphospholipid Syndrome Boards:

Pregnancy with Antiphospholipid Syndrome
Pregnancy is a tricky and touchy topic when it comes to Antiphospholipid Syndrome. I’ve known people who have had miscarriages at 8 months, because they didn’t know that they had APS. The incidence of pregnancy loss for women with Antiphospholipid Syndrome is reported to be between 34% – 76% (Xu et al., 2019).
Women with APS need to work closely with their rheumatologist, a high-risk gynaecologist, and their entire healthcare team – before even getting pregnant. Your healthcare team should assess your risk factors, and work with you to implement preventive strategies, as well as come up with a plan that is tailored to your specific circumstances and health status (Andreoli et al., 2017).
So Why Not Screen Every Woman Before Pregnancy for Antiphospholipid Antibodies?
I used to think that every woman should be tested for APS before pregnancy to save us the grief of a miscarriage. But having done more research, I can understand why it isn’t such a good idea to do so. In general, asymptomatic patients should not be screened for aPLs as there is a risk of false positivity of up to 3 – 20% (Rand and Wolgast, 2012). This not only subjects the individual to unnecessary treatment, but increases the risk of bleeding complications.
Before screening for aPLs, the patient’s medical history (such as recurrent miscarriages), as well as comorbidities (such as SLE), need to make sense medically too. False positive aPLs tests can also be triggered by many types of infections, such as Lyme Disease, Syphilis and EBV (Rand and Wolgast, 2012).
Will I Pass Antiphospholipid Syndrome on to My Child?
Like many other autoimmune diseases, Antiphospholipid Syndrome is polygenic, where multiple genes are involved, and can also be influenced by environmental factors. The individual contribution of each gene may even be unnoticeable, and requires multiple contributing factors to activate (Lvovs et al., 2012). Whilst yes, the genes are still there, APS is not commonly inherited from parent to child, as compared to other conditions such as sickle cell anaemia or cystic fibrosis.
According to Barinotti et al. (2020):
“The etiology of APS is still unknown, but similarly to other autoimmune diseases, it seems to be linked to a complex interplay between genetic predisposition, antigenic stimuli, and the presence of specific autoantibodies.” ….. “APS is a complex rare disease with the great majority of the cases being sporadic. Rarely, the condition has been reported to run in families.”
There is still much to be understood as to how Antiphospholipid Syndrome ultimately develops, but according to Ortiz-Fernández and Sawalha (2019), recent studies have identified some of the genetic components that contribute to APS, such as: “antigen receptor-mediated signaling, interferon-gamma-mediated signaling, T cell receptor signaling, and regulation of B cell receptor signaling pathways”. Genetic susceptibility to APS is also dependent on ethnic, gene-gene, gene-environment, and a multitude of other factors that are still not completely understood (Horita and Merrill, 2004).
Medication Interactions During Pregnancy with Antiphospholipid Syndrome
It is important to inform your medical team before even trying for pregnancy, as certain medications that you’re on might be harmful to either yourself or the foetus. Some medications take a while to be completely eradicated from your bodily system as well. Your doctor will usually switch you to a safer alternative for pregnancy, and monitor you closely for adverse effects.
Here are some important medications to take note of in relation to APS and pregnancy. If you have other medications that you take for other chronic illnesses, they should also be taken into account.
Warfarin & Pregnancy
Warfarin medication should be paused during pregnancy, as it can be harmful to foetuses. Apart from a rare disease called Foetal Warfarin Syndrome (Starling et al., 2012), warfarin can also cause other complications during pregnancy such as developmental problems or haemorrhages (Abadi et al., 2002).
Hydroxychloroquine, Low Molecular Weight Heparin (LMWH) & Aspirin in Pregnant APS Patients
Recent studies have also shown that the current standard for pregnancy in APS patients, which uses aspirin and LMWH, are limited in efficacy of late-term pregnancy complications. Interestingly, hydroxychloroquine, which is an anti-malarial drug commonly used to treat Lupus (SLE), showed benefits for APS patients in pregnancy.
A study on 176 pregnancies (96 with aPLs) reflected a higher rate of live births, and a lower prevalence of aPLs-related pregnancy morbidity whilst on hydroxychloroquine (Sciascia et al., 2016). Another European multicentre study of 35 APS patients who were put on hydroxychloroquine, showed a decrease in pregnancy losses from 81% to 19%, as compared to previous usage of aspirin and/or LMWH only (Mekinian, 2015).
These findings by Marchetti et al. (2014) explain why hydroxychloroquine may be beneficial for APS patients who are pregnant:
“Here, we observed that HCQ completely reversed the effects of the plasma of APS patients (free from anti–annexin V antibodies) and the anti‐β2GP1 antibodies on trophoblastic cell fusion and differentiation.”
Something interesting I found whilst doing research on this topic, is that in Japan, APS-complicated pregnancies from the usage of LMWH is not covered by medical insurance. Thus, unfractionated heparin (UFH) and/or low-dose aspirin (LDA) are used instead (Deguchi et al., 2017). In addition, there is also something known as aspirin-heparin resistant APS (AHRAPS), where other therapies such as high-dose intravenous immunoglobulin (IVIG) may need to be included (Arumugham and Rayi, 2023).
Should you be interested to learn more, this paper by Schreiber and Hunt (2019) does an excellent job of listing out the management of APS during pregnancy. You can also learn more about warfarin, LMWH and other medications related to APS in this post.
-
Read Related Posts:
- Why Painkillers are One of My Biggest Allies for a Decent Quality of Life
- After Surgery Care at Home: Hygiene Resources
- Wound Care & What to Wear After Knee Surgery
- An Anaphylaxis Reaction from Rituximab in Between Shady Years
Pin to Your Women’s Health in Antiphospholipid Syndrome Boards:

Pregnancy Complications with Antiphospholipid Syndrome
Whilst it is important not to worry yourself sick(er), it is also important to be aware of potential pregnancy complications as a result of Antiphospholipid Syndrome, in order to better care and advocate for yourself. These complications can arise from a combination of factors – from the disease itself, comorbidities, hormonal changes, decreased mobility, stage of pregnancy, and method of delivery, with caesarean section carrying a higher risk of thrombosis than vaginal delivery (Lee et al., 2021).
Whilst there are certain guidelines sketched out for the management of APS during pregnancy, more studies still need to be done in order to determine the best course of action. Thus, it is critical to ensure that your pregnancy care is individualised based on your medical history, comorbidities such as SLE, lifestyle factors and more.
Trophoblasts
Pregnancy complications do not just stem from a single factor; Antiphospholipid Syndrome can attack from different pathways. For one, they can interact with trophoblasts, which interfere with nutrients transmitted to the embryo in the placenta. Apart from nutrients, trophoblasts also produce numerous growth factors and hormones that support healthy foetal and placental development (Wang and Zhao, 2010).
According to a study by Mulla et al. (2009):
“Our findings suggest that early pregnancy loss and late obstetric complications in APS may arise from anti-β2GPI Abs acting on first trimester trophoblast cells, thereby triggering placental inflammation and cell death.”
Meaning to say that antiphospholipid antibodies trigger an inflammatory response in the placenta, which interferes with trophoblasts. Studies have found that hydroxychloroquine, an anti-malarial drug commonly used to treat Lupus (SLE), has beneficial effects against this.
Preeclampsia
Preeclampsia is also associated with Antiphospholipid Syndrome, and can also be associated with shallow cytotrophoblast invasion (Skoura et al., 2022). Preeclampsia manifests as hypertension and sometimes with protein in the urine (proteinuria). It is vital to work with your obstetrician closely, as this can be a deadly condition.
Venous Thromboembolism (VTE)
Another severe complication of pregnancy for APS patients is venous thromboembolism (VTE), especially in the legs (DVTs). This can manifest systemically with mild to life-threatening symptoms, from pain and swelling, to blood clots in the lungs (pulmonary embolism). Left-sided DVTs are also more common at 70% – 90%, due to the involvement of certain veins during pregnancy. VTE is also still a risk during the postpartum period (Varrias et al., 2023).
In general, LMWH is used for the prevention and treatment of VTEs in pregnant women, although that depends on the individual as well. Here are some guidelines based on observational studies and extrapolation from Bates et al. (2012).
Read more about cardiovascular and pulmonary embolisms in general relation to APS here.
Intrauterine Growth Restriction (IUGR)
Intrauterine Growth Restriction (IUGR) – also known as Foetal Growth Restriction – is another pregnancy complication with APS, where the foetus is estimated to be below the 10th percentile for its gestational age. Women with APS were found to have a higher rate of IUGR, with anticardiolipin (aCL) positivity highly associated with it (Xi et al., 2020).
In another systematic meta-analysis, anticardiolipin antibodies (aCLs) and anti-beta2 glycoprotein 1 antibodies (anti-β2GP1) were also found to be associated with IUGR, whilst lupus anticoagulant (LA) did not increase the chances of it (Xu et al., 2022). This further demonstrates the complexity of antiphospholipid antibodies and APS in general, as each type of antibody affects the body differently, yet are all indicators towards an Antiphospholipid Syndrome diagnosis.
Pin to Your Female, Autoimmune Disease & APS Boards:

Miscarriages & Antiphospholipid Syndrome
Sadly, many women only discover that they have Antiphospholipid Syndrome after recurrent miscarriages. According to Di Prima et al. (2011):
“Obstetric complications are the hallmark of antiphospholipid syndrome. Recurrent miscarriage, early delivery, oligohydramnios, prematurity, intrauterine growth restriction, Foetal distress, Foetal or neonatal thrombosis, pre-eclampsia/eclampsia, HELLP syndrome, arterial or venous thrombosis and placental insufficiency are the most severe APS-related complication for pregnant women.”
Foetal loss over 10 weeks of gestation is more common in women with Antiphospholipid Syndrome, although about half of recurrent miscarriages occur during the first trimester. The lupus anticoagulant also plays a big role in recurrent miscarriages before the 24th week of gestation (Di Prima et al., 2011).
The rate of successful births is more than 80% although there are still risks, such as preeclampsia and preterm delivery (Abrahams et al., 2017). I can’t emphasise this enough, but it is extremely important to work closely with a high-risk obstetrician and your entire healthcare team throughout your pregnancy journey.
The Stigma Associated with Women’s Health in Antiphospholipid Syndrome, and the Perceived Inability to Conceive
I have always known that I want to be a mother – since I was 14 in fact. In my young teenage mind, I would get married at 27, have a few children, and devote myself to bringing them up as well as I can. I was prepared to suffer through all pains and risks to do so. Unfortunately, life doesn’t work that way. It is not to be dictated – it sets the direction and pace, and you can either choose to adapt, or struggle double.
I am now 38, with neither partner nor child. My biological clock is ticking, but there is nothing I can do about it. After all, marriage and/or pregnancy takes two hands to clap. Partners and potential partners have also written me off due to my multitude of chronic illnesses; they do not even want to begin with the slightest possibility of me being unable to conceive.
This is a phenomenological study that might resonate with you, if you have experienced foetal loss as a woman with Antiphospholipid Syndrome (Mahmoud et al., 2020). It illustrates the social burdens felt by patients, with some marriages ending in divorce due to expectations from husbands and mother-in-laws. The psychological suffering of the patient is huge as well, with many women feeling sad and frustrated. Some choose to delay subsequent pregnancies, and many live in constant fear due to the unpredictability.
It is important for healthcare professionals to educate their patients on potential complications, from the physical to emotional. In my opinion, it is also the responsibility of loved ones to educate themselves on women’s health in Antiphospholipid Syndrome, and to understand that it is not their partner’s fault. As a patient, know that you are not alone, and that you are not to be blamed.
-
Read Related Posts:
- Would You Date a Person with Chronic Illness?
- 4 Cool Truths My (Ex) Partner Said (Unwittingly)
- Loss of Identity with Chronic Illness & The Plot Twist: Sharpened Self-Worth
- Sometimes, Physical Pain Isn’t the Worst Part About Chronic Illness
- Life with Chronic Illness: Happiness & Pain Can Co-exist
Ongoing Research on Recurrent Miscarriages in APS
A systematic review and meta-analysis has been done to try and determine which antiphospholipid antibodies play a bigger role in recurrent miscarriages for female patients with APS. Whilst it has been found that anticardiolipin antibodies, lupus anticoagulant, anti-β2-glycoprotein I antibodies and antiphosphatidylserine (which is just about all of them…) are related to recurrent miscarriages, more studies still need to be done to learn more about what they truly do (da Silva Santos et al., 2017).
Up to 15% of unexplained stillbirths might be due to aPLs (antiphospholipid antibodies), with women with lupus anticoagulant or who are ‘triple apL positive’ at the highest risk (Herrera et al., 2017).
Menopause and Antiphospholipid Syndrome
There are not many studies done on APS and menopause but in general, menopause is a period of immune changes within the female body, amongst other events. Levels of oestrogen and DHEA sulfate decrease, which may lead to: an increase in proinflammatory cytokines, a decrease in certain anti-inflammatory cytokines, decreased lymphocyte levels (CD4+ T cells and B cells), and a decrease in cytotoxic activity of NK cells (Bove, 2013).
Depending on the individual, type of autoimmune diseases they have, and other factors such as epigenetics and environment, the menopause transition can occur quite differently. There are some reports of decreased frequency of pain flares in patients with SLE, yet at the same time, greater damage accrual in affected organs (Bove, 2013).
In one controlled study, premenopausal women with APS/SLE were also found to have an increased risk of atherosclerosis, which is a buildup of fats, cholesterol and other substances in and on the artery walls (Vlachoyiannopoulos et al., 2003). As per Mayo Clinic, whilst atherosclerosis is often considered a heart problem, it can also occur in any other artery in the human body. The plaque not only cause the arteries to narrow, they may also burst and lead to blood clots. Learn more about cardiovascular manifestations in APS patients here.
Conclusion to Women’s Health in Antiphospholipid Syndrome
I hope that this article provides you with the added knowledge to better care for yourself as a female with Antiphospholipid Syndrome. From menstruation to child birth to menopause – the journey of womanhood is intertwined with blood, so it’s vital to be aware of how APS can interact with each stage of your life.
Should you have any questions, corrections, experiences, or more knowledge to share – feel free to leave a comment below so we can all learn together. Don’t forget to check out the other posts in the series listed below, too!
I have spent countless hours working on these articles. If you appreciate what I do and would like to show your support, you can do so via the button below (absolutely no obligations, though!) 
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Antiphospholipid Syndrome Diagnosis: The A to Z Guide as a Patient
- Latest Research on Antiphospholipid Syndrome (2024 Edition)
- The Lowdown on Medications & Antiphospholipid Syndrome (Warfarin, Enoxaparin, DOACs, NSAIDs & More)
- How Does Antiphospholipid Syndrome Affect The Body? (Beyond the Blood to Major Organs)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
Pin to Your Women’s Health in Antiphospholipid Syndrome Boards:
- Abadi, S., Einarson, A., & Koren, G. (2002). Use of warfarin during pregnancy. Canadian Family Physician, 48(4), 695-697. Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/12046363
- Abou-Ismail, M. Y., Sridhar, D. C., & Nayak, L. (2020). Estrogen and thrombosis: a bench to bedside review. Thrombosis research, 192, 40-51. https://doi.org/10.1016/j.thromres.2020.05.008
- Abrahams, V. M., Chamley, L. W., & Salmon, J. E. (2017). Emerging Treatment Models in Rheumatology: Antiphospholipid Syndrome and Pregnancy: Pathogenesis to Translation. Arthritis & Rheumatology, 69(9), 1710–1721. https://doi.org/10.1002/art.40136
- Andreoli, L., Bertsias, G. K., Agmon-Levin, N., Brown, S., Cervera, R., Costedoat-Chalumeau, N., Doria, A., Fischer-Betz, R., Forger, F., Moraes-Fontes, M. F., Khamashta, M., King, J., Lojacono, A., Marchiori, F., Meroni, P. L., Mosca, M., Motta, M., Ostensen, M., Pamfil, C., … Tincani, A. (2017). EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Annals of the Rheumatic Diseases, 76(3), 476–485. https://doi.org/10.1136/annrheumdis-2016-209770
- APS Support UK. (n.d.). APS and Women’s Health. APS Support UK. Retrieved from: https://aps-support.org.uk/self-help/living-with-aps/aps-and-womens-health
- Arumugham, V. B., & Rayi, A. (2023). Intravenous immunoglobulin (IVIG). In StatPearls [Internet]. StatPearls Publishing. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK554446/
- Bates, S. M., Greer, I. A., Middeldorp, S., Veenstra, D. L., Prabulos, A. M., & Vandvik, P. O. (2012). VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest, 141(2), e691S–e736S. https://doi.org/10.1378/chest.11-2300
- Barinotti, A., Radin, M., Cecchi, I., Foddai, S. G., Rubini, E., Roccatello, D., Sciascia, S., & Menegatti, E. (2020). Genetic Factors in Antiphospholipid Syndrome: Preliminary Experience with Whole Exome Sequencing. International journal of molecular sciences, 21(24), 9551. https://doi.org/10.3390/ijms21249551
- Bove, R. (2013). Autoimmune diseases and reproductive aging. Clinical Immunology, 149(2), 251-264. https://doi.org/10.1016/j.clim.2013.02.010
- Cleveland Clinic. (2023). Hemoperitoneum. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/hemoperitoneum
- Cleveland Clinic. (2022). Intrauterine Device (IUD). Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/treatments/24441-intrauterine-device-iud
- Cleveland Clinic. (2022). Intrauterine Growth Restriction. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/24017-intrauterine-growth-restriction
- Cleveland Clinic. (n.d.). Etonogestrel Implant. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/drugs/18407-etonogestrel-implant
- Cunha, J. P. (Ed.). (2022). Implanon. RxList. Retrieved from: https://www.rxlist.com/implanon-drug.htm
- Dabit, J. Y., Valenzuela-Almada, M. O., Vallejo-Ramos, S., & Duarte-García, A. (2021). Epidemiology of antiphospholipid syndrome in the general population. Current rheumatology reports, 23(12), 85. https://doi.org/10.1007/s11926-021-01038-2
- da Silva Santos, T., Ieque, A. L., de Carvalho, H. C., Sell, A. M., Lonardoni, M. V. C., Demarchi, I. G., … & Teixeira, J. J. V. (2017). Antiphospholipid syndrome and recurrent miscarriage: A systematic review and meta-analysis. Journal of reproductive immunology, 123, 78-87. https://doi.org/10.1016/j.jri.2017.09.007
- Deguchi, M., Yamada, H., Sugiura-Ogasawara, M., Morikawa, M., Fujita, D., Miki, A., … & Murashima, A. (2017). Factors associated with adverse pregnancy outcomes in women with antiphospholipid syndrome: a multicenter study. Journal of reproductive immunology, 122, 21-27. https://doi.org/10.1016/j.jri.2017.08.001
- Delgado, B. J., & Lopez-Ojeda, W. (2023). Estrogen. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK538260/
- Di Prima, F. A., Valenti, O., Hyseni, E., Giorgio, E., Faraci, M., Renda, E., De Domenico, R., & Monte, S. (2011). Antiphospholipid Syndrome during pregnancy: the state of the art. Journal of prenatal medicine, 5(2), 41–53. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3279165/
- Dou, D. R., Zhao, Y., Belk, J. A., Zhao, Y., Casey, K. M., Chen, D. C., … & Chang, H. Y. (2024). Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell, 187(3), 733-749. https://doi.org/10.1016/j.cell.2023.12.037
- Graham, F. (2024). Daily briefing: Autoimmune disease linked to X chromosome. Nature. Retrieved from: https://www.nature.com/articles/d41586-024-00342-y
- Harding, A. T., & Heaton, N. S. (2022). The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers, 14(4), 909. https://doi.org/10.3390/cancers14040909
- Herrera, C. A., Heuser, C. C., & Branch, D. W. (2017). Stillbirth: the impact of antiphospholipid syndrome?. Lupus, 26(3), 237-239. https://doi.org/10.1177/0961203316671815
- Hindorff, L. (2024). Polygenic Trait. National Human Genome Research Institute. Retrieved from: https://www.genome.gov/genetics-glossary/Polygenic-Trait
- Horita, T., & Merrill, J. T. (2004). Genetics of antiphospholipid syndrome. Current rheumatology reports, 6(6), 458–462. https://doi.org/10.1007/s11926-004-0025-0
- Jara, L. J., Medina, G., Vera-Lastra, O., & Barile, L. (2005). The impact of gender on clinical manifestations of primary antiphospholipid syndrome. Lupus, 14(8), 607-612. https://doi.org/10.1191/0961203305lu2176oa
- Kaul, A., Jawad, A., & Hughes, G. (2023). Antiphospholipid syndrome, thrombosis and multidisciplinary management. Trends in Urology & Men’s Health, 14(3), 31-35. https://doi.org/10.1002/tre.911
- Lee, E. E., Jun, J. K., & Lee, E. B. (2021). Management of women with antiphospholipid antibodies or antiphospholipid syndrome during pregnancy. Journal of Korean Medical Science, 36(4). https://doi.org/10.3346/jkms.2021.36.e24
- Lvovs, D., Favorova, O. O., & Favorov, A. V. (2012). A Polygenic Approach to the Study of Polygenic Diseases. Acta naturae, 4(3), 59–71. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3491892/
- Mekinian, A., Lazzaroni, M. G., Kuzenko, A., Alijotas-Reig, J., Ruffatti, A., Levy, P., … & Fain, O. (2015). The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmunity reviews, 14(6), 498-502. https://doi.org/10.1016/j.autrev.2015.01.012
- Mahmoud, F. Z., Elsayed, Y. A., & Eswi, A. S. (2020). The Lived Experience of Hospitalized Pregnant Women Having Antiphospholipid Syndrome with a Previous Fetal Loss: A Phenomenological Study. Rehabilitation, 5(1), 5-11. https://doi.org/10.11648/j.rs.20200501.12
- Manukyan, G., Martirosyan, A., Slavik, L., Ulehlova, J., Dihel, M., Papajik, T., & Kriegova, E. (2020). 17β-Estradiol Promotes Proinflammatory and Procoagulatory Phenotype of Innate Immune Cells in the Presence of Antiphospholipid Antibodies. Biomedicines, 8(6), 162. https://doi.org/10.3390/biomedicines8060162
- Marchetti, T., Ruffatti, A., Wuillemin, C., De Moerloose, P., & Cohen, M. (2014). Hydroxychloroquine restores trophoblast fusion affected by antiphospholipid antibodies. Journal of Thrombosis and Haemostasis, 12(6), 910-920. https://doi.org/10.1111/jth.12570
- Mayo Clinic. (2023). Ovarian cysts. Mayo Clinic. Retrieved from: https://www.mayoclinic.org/diseases-conditions/ovarian-cysts/symptoms-causes/syc-20353405
- Mayo Clinic. (2022). Arteriosclerosis / atherosclerosis. Mayo Clinic. Retrieved from: https://www.mayoclinic.org/diseases-conditions/arteriosclerosis-atherosclerosis/symptoms-causes/syc-20350569
- Mulla, M. J., Brosens, J. J., Chamley, L. W., Giles, I., Pericleous, C., Rahman, A., … & Abrahams, V. M. (2009). Antiphospholipid antibodies induce a pro‐inflammatory response in first trimester trophoblast via the TLR4/MyD88 pathway. American Journal of Reproductive Immunology, 62(2), 96-111. https://doi.org/10.1111/j.1600-0897.2009.00717.x
- NHS. (2022). Pregnancy, breastfeeding and fertility while taking warfarin. NHS. Retrieved from: https://www.nhs.uk/medicines/warfarin/pregnancy-breastfeeding-and-fertility-while-taking-warfarin/
- Ortiz-Fernández, L., & Sawalha, A. H. (2019). Genetics of Antiphospholipid Syndrome. Current Rheumatology Reports, 21(12), 65. https://doi.org/10.1007/s11926-019-0869-y
- Rand, J. H., & Wolgast, L. R. (2012). Dos and don’ts in diagnosing antiphospholipid syndrome. Hematology 2010, the American Society of Hematology Education Program Book, 2012(1), 455-459. https://doi.org/10.1182/asheducation.V2012.1.455.3806865
- Rosendaal, F. R., Helmerhorst, F. M., & Vandenbroucke, J. P. (2002). Female hormones and thrombosis. Arteriosclerosis, thrombosis, and vascular biology, 22(2), 201-210. https://doi.org/10.1161/hq0202.102318
- Sammaritano, L. R. (2021). Which hormones and contraception for women with APS? Exogenous hormone use in women with APS. Current Rheumatology Reports, 23(6), 44. https://doi.org/10.1007/s11926-021-01006-w
- Schreiber, K., & Hunt, B. J. (2019). Managing antiphospholipid syndrome in pregnancy. Thrombosis research, 181, S41-S46. https://doi.org/10.1016/S0049-3848(19)30366-4
- Sciascia, S., Hunt, B. J., Talavera-Garcia, E., Lliso, G., Khamashta, M., & Cuadrado, M. J. (2016). The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. American journal of obstetrics and gynecology, 214(2), 273-e1. https://doi.org/10.1016/j.ajog.2015.09.078
- Skoura, R., Andronikidi, P. E., Anestakis, D., Petanidis, S., Orovou, E., Tzitiridou, M., & Eskitzis, P. (2022). Antiphospholipid Syndrome and Preeclampsia in Pregnancy: A Case Report. Cureus, 14(8), e28458. https://doi.org/10.7759/cureus.28458
- Starling, L. D., Sinha, A., Boyd, D., & Furck, A. (2012). Fetal warfarin syndrome. Case Reports, 2012, bcr2012007344. https://doi.org/10.1136/bcr-2012-007344
- Varrias, D., Spanos, M., Kokkinidis, D. G., Zoumpourlis, P., & Kalaitzopoulos, D. R. (2023). Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vascular health and risk management, 19, 469–484. https://doi.org/10.2147/VHRM.S404537
- Vlachoyiannopoulos, P. G., Kanellopoulos, P. G., Ioannidis, J. P. A., Tektonidou, M. G., Mastorakou, I., & Moutsopoulos, H. M. (2003). Atherosclerosis in premenopausal women with antiphospholipid syndrome and systemic lupus erythematosus: a controlled study. Rheumatology, 42(5), 645-651. https://doi.org/10.1093/rheumatology/keg182
- Wang, Y., & Zhao, S. (2010). Chapter 4 – Cell types of the placenta. In: Vascular biology of the placenta. Morgan & Claypool Life Sciences. Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK53245/
- Xi, F., Cai, Y., Lv, M., Jiang, Y., Zhou, F., Chen, Y., … & Luo, Q. (2020). Anticardiolipin positivity is highly associated with intrauterine growth restriction in women with antiphospholipid syndrome. Clinical and Applied Thrombosis/Hemostasis, 26, 1076029620974455. https://doi.org/10.1177/1076029620974455
- Xu, J., Chen, D., Tian, Y., Wang, X., & Peng, B. (2022). Antiphospholipid Antibodies Increase the Risk of Fetal Growth Restriction: A Systematic Meta-Analysis. International journal of clinical practice, 2022, 4308470. https://doi.org/10.1155/2022/4308470
- Xu, J., Chen, D., Duan, X., Li, L., Tang, Y., & Peng, B. (2019). The association between antiphospholipid antibodies and late fetal loss: A systematic review and meta‐analysis. Acta obstetricia et gynecologica Scandinavica, 98(12), 1523-1533. https://doi.org/10.1111/aogs.13665
- Yu, R. Z. (2021). Is Antiphospholipid Syndrome (APS) Hereditary? If I Have APS, Should My Family Members Be Tested? Michigan Medicine, University of Michigan. Retrieved from: https://medicine.umich.edu/dept/intmed/antiphospholipid-syndrome-aps-hereditary-if-i-have-aps-should-my-family-members-be-tested
References:
14 Summer Essentials to Keep You Cool and Protected
Whether you’re young or old, heat sensitivity can cause extreme discomfort in the summer months. With the right products, you can still enjoy the season doing the activities you enjoy. In this article we’ll show you how to beat the heat with these Amazon summer […]
mind-&-emotionsWhether you’re young or old, heat sensitivity can cause extreme discomfort in the summer months. With the right products, you can still enjoy the season doing the activities you enjoy. In this article we’ll show you how to beat the heat with these Amazon summer essentials.
This post contains affiliate links. If you make a purchase through a link, I may receive a small commission, at no cost to you.

Heat Intolerance versus Photosensitivity
Summer heat can be hard enough to deal with when you’re in good health. But for some, medical conditions like multiple sclerosis and hyperthyroidism can cause heat intolerance. Warm temperatures can cause excessive sweating, dizziness, fatigue, nausea, vomiting, and even heat stroke.
Even menopause can cause an inability to tolerate warm temperatures. Add to that hot flashes, night sweats, and increased sweating, and you’re like a walking sauna.
Other conditions like lupus, Sjogren’s Syndrome, dermatomyositis and even certain medicines can cause photosensitivity. Exposure to the UV rays of the sun causes the skin to react. Itching, redness, rashes, blisters, hyperpigmentation, and severe sunburn can result.
Summer Essentials to Keep You Cool and Protected
The products below have been selected for their ability to do one or more of the following:
- Cool the body when exposed to warm temperatures
- Provide soothing relief/protection from UV rays
Products to Cool You Down

Portable Handheld Fans
Get instant cooling on those hot summer days. This handheld fan is lightweight and small enough to fit in your pocketbook. These fans are a lifesaver in crowded environments. And for my “mature” (a.k.a. menopausal) crowd, they’re perfect to use all year long. I even keep one by my bedside to use at night!
Handheld Misting Fans
These portable fans provide cool air and a mist spray to cool you down with a quickness. Great for outdoor events. This rechargeable fan has 3 speeds. It’s simple design makes it easy to turn off and on with one touch.
Portable Neck Fans
This hands-free unit sits comfortably around the neck. And because there are no rotating blades, it’s perfect for children and adults of all ages. Not to mention hair can’t get caught in it. This is a great companion for outdoor activities like gardening and grilling.
Cooling Towels
Manage body temperature and get instant relief from summer heat with these cooling towels. Simply wet and wring out excessive water. These Microfiber towels retain moisture for up to 3 hours after wetting. They’re perfect to use for heat stroke prevention or even headache therapy. And they come with a pouch to make carrying easy.
Best Products to Stay Hydrated
Insulated Water Bottles
Staying hydrated is a must to help regulate body temperature. Water is needed to produce sweat. When sweat evaporates, the body cools down. Insulated water bottles keep your water cool for hours. These stainless steel water bottles come with a straw, handle, and strap for easy carrying.
Electrolyte Drink Packets
Stay hydrated and replenish electrolytes lost through sweating with these convenient powder packets. Simply mix with 8 ounces of water. They come in 4 delicious flavors and provide 3 times the electrolytes and half the sugar as other sports drinks. And they’re gluten-free, non-GMO, and don’t include artificial preservatives.
Summer Essentials to Protect Skin From Sun

Beach Umbrella
This product is an absolute beach necessity. Don’t get caught without one only to have to rent one on the beach for an exorbitant price. This heavy duty 7-foot umbrella comes with a carrying case, a built in table, and a sand anchor. Not to mention the umbrella tilts and its material provides 99% UV protection.
Portable Sun Shelter
If you’re looking for broader protection, this sun shelter with its 9×9 foot canopy has you covered (pun intended). Like the umbrella above, this sun shelter provides 99% UV protection. It also comes with built in sandbags that can be easily adjusted to provide better coverage as the sun travels. Great for the beach, camping, and outdoor fun!
Recliner Chair with Umbrella
Lay back and relax with this portable recliner with adjustable umbrella. Or simply sit up. This lightweight recliner gives you 3 options. Like the 2 products above, the canopy provides UV protection. This recliner also Includes a cup holder and enough insulated storage to fit 4 drinks. Perfect for picnics, beach outings, or park visits.
Protective Clothes

When performing outdoor activities, the American Academy of Dermatology recommends covering as much skin as possible. Opt for lightweight, tightly woven shirts and pants. In other words, avoid loosely weaved fabrics like lace. Look for fabrics that offer UV protection. Bear in mind, dark colors provide greater UV protection than light colors. Clothes that absorb moisture are best since dry clothing offers greater sun protection. Here are just a few styles to choose from.
Quick Dry Long Sleeve Sun Shirts
Long Sleeve Sun Protection Shirts with Rash Guard
You May Also Like
Ultimate Guide to Traveling With Chronic Pain
50 Best Self-Care Gifts for Women for Relaxation and Well-Being
My 15 Essential Chronic Illness Must Haves: Products to Enhance Your Quality of Life
Arch Support Flip Flops
Did you know you can actually burn your feet in the sand? This is especially the case for people with neuropathy resulting from diabetes. This condition makes it difficult to detect how hot the sand is. Also people with thin skin from conditions like eczema and psoriasis may burn easily. Protect your feet from the hot sand while supporting your arches. These aren’t ordinary flip flops, ladies. These textured slip-ons have a yoga mat insole and sturdy footbed. They’re perfect for people with painful conditions like foot pain, arch pain, and plantar fasciitis. Check out these fun colors.
Protective Hats

Minimize sun exposure with a protective hat. Ideally, you want to choose something with a wide brim made of breathable materials. Adjustable straps and drawstrings are good for breezy days. Also, look for hats with a UPF rating of 50 or higher. Here are just a few styles that you can choose from that received good reviews on Amazon.
Foldable Cool Mesh Ponytail Bucket Hat
Sunscreen for Sensitive Skin
No summer essentials is complete without sunscreen. While everyone should wear sunscreen, certain conditions are especially sensitive to UV rays. At the same time conditions where the skin barrier is compromised like eczema and psoriasis may react to certain ingredients. CeraVe has a hydrating mineral sunscreen for face and body that has an SPF of 50 and is free of oil and fragrances.
Summer Essentials to Keep You Cool At Night

Cooling Sheets
These sheets are designed to keep “hot sleepers” cozy. They’re made from a proprietary material that keeps you cool and dry while pulling moisture away from the body. And they come in a variety of sizes including twin, twin XL, full, queen, king and California king.
Cooling Pillows
Sleep longer and deeper with pillows designed to regulate body temperature. By keeping head, neck and shoulders cool, these memory foam pillows help to prevent night sweats. And they’re perfect for back, stomach and side sleepers.
Summary
Just because you live with a chronic condition doesn’t mean you have to can’t enjoy summer. Staying hydrated, keeping cool, and protecting your skin from the UV rays has never been easier. You can enjoy the beach, camping, picnicking, or your favorite summer activity. Stay comfortable and safe with these summer essentials.
.
The post 14 Summer Essentials to Keep You Cool and Protected appeared first on Health Uprising Now.

Latest Research on Antiphospholipid Syndrome (2024 Edition)
The bad news about Antiphospholipid Syndrome (APS) is that it’s chronic. The good news is that there are quite a number of exciting new treatments in the works. This article is part of the APS resource library that I’m building up on the site for […]
Chronic voicesThe bad news about Antiphospholipid Syndrome (APS) is that it’s chronic. The good news is that there are quite a number of exciting new treatments in the works. This article is part of the APS resource library that I’m building up on the site for patients, as a patient who has lived with it for more than 20 years myself.
This post will focus on the latest research on Antiphospholipid Syndrome as of 2024, and I aim to update it as frequently as I can. The ultimate A to Z resource guide on Antiphospholipid Syndrome has also been released, so don’t forget to check that out for more in-depth information about APS.
*Disclaimer: This article is meant for educational purposes, and is based on my personal experiences as a patient. Whilst I have done my utmost to be meticulous in research, I am not a doctor, and nothing in this article should be substituted for medical advice. Please consult your own doctor before changing or adding any new treatment protocols. This post may also contain affiliate links. It will cost you nothing to click on them. I will get a small referral fee from purchases you make, which helps with the maintenance of this blog. Read our Privacy Policy page for more information. Thank you!
-
Last Updated:
- 7 June 2024: Discovery of 2 Missing Enzymes in People with Antiphospholipid Syndrome
-
Read Related Posts in the Antiphospholipid Syndrome Diagnosis Series:
- Antiphospholipid Syndrome Diagnosis: The A to Z Guide as a Patient
- Pregnancy, Miscarriage & Women’s Health in Antiphospholipid Syndrome
- The Lowdown on Medications & Antiphospholipid Syndrome (Warfarin, Enoxaparin, DOACs, NSAIDs & More)
- How Does Antiphospholipid Syndrome Affect The Body? (Beyond the Blood to Major Organs)
- The Annoying Thing About Living with Antiphospholipid Syndrome (My Personal Experiences)
- An Experience from Hell: Pulmonary Embolism, DVTs & Antiphospholipid Syndrome
- What it Feels Like to be Refused Treatment by a Hospital’s A&E / ER
Pin to Your Antiphospholipid Syndrome & Autoimmune Disease Boards:

Personal Research is Important as a Patient
Before I begin, I just wanted to emphasise that it is important that you do your own research as a patient with Antiphospholipid Syndrome. This can feel like a colossal task in the beginning, with a long list of foods, drinks, medications and activities that you need to moderate. It took me a long time to figure out what works for me through trial and error, often unwittingly. But trust me, you will learn over time, and become more confident with living with this autoimmune disease. Knowledge is power, and doing your own research on Antiphospholipid Syndrome in relation to its impact on your personal life can also help to make you feel more empowered.
“Knowledge is power, and doing your own #research on #AntiphospholipidSyndrome in relation to its impact on your personal life can also help to make you feel more #empowered.” #ChronicIllness #APS
Share on X
There are also a few things you can do online to ensure that you don’t miss out on the latest research on Antiphospholipid Syndrome. I personally subscribe to Google Alerts for keywords related to: “Antiphospholipid Syndrome”, “Lupus”, “autoimmune disease”, “chronic illness” and the likes.
Another tool I use is Feedly, an RSS (Really Simple Syndication) feed, where I subscribe to specific websites and news to browse through on a daily basis. If you’re curious as to how an RSS feed looks like, this is the one for this website. You simply need to add the URL to Feedly, and everything published on my blog will be in your reader. The best part is that these tools are free to use, and you can add any other topic that you’re interested in as well!
Finally, I also rely quite a bit on Google Scholar to learn more about various aspects of Antiphospholipid Syndrome from a medical and scientific perspective. In fact, all of the latest research on Antiphospholipid Syndrome that you will read about in this post can be found on Google Scholar – so it is indeed possible to keep up with the research on your own, too! There are many websites on ‘regular’ Google search, but it’s important to note that not all of these sources are verified, whereas articles on Google Scholar are. If you do choose to do your own research via ‘regular’ Google, do ensure that the source is from an established organisation, such as the NHS or Cleveland Clinic.
A Breakdown of the Latest Research on Antiphospholipid Syndrome
Whilst there are quite a number of promising new research and clinical trials for Antiphospholipid Syndrome, it is still a rare disease that could use more public awareness and research funding. The American College of Rheumatology (ACR) holds an annual convergence that showcases the latest research and knowledge into all areas of rheumatology. Here are the new Antiphospholipid Syndrome findings that were presented at the ACR convergence in 2023, and I can’t wait to see what will be shared in the next one in November 2024!
“Check out the latest #research on #AntiphospholipidSyndrome, from diagnosis tools to proteins, NETs, DNA molecules and more.” #APS #AutoimmuneDisease #ChronicIllness
Share on X
Pin to Your Antiphospholipid Syndrome & Research Boards:

Peptide Libraries for Diagnosing Antiphospholipid Syndrome
Let’s begin with exciting new advancements in the diagnosis stage of APS. The current tests available for the detection of autoantibodies that target prothrombin (aPT) are aPS/PT, and aPT-A assays. However, it has been difficult to standardise their detection in lab tests, due to variability in platforms and protocols.
One new diagnostic tool in the works is a novel ELISA assay, ProTS525A-Biot (aPT-Bio). In a study of 27 high-risk APS patients, the ProTS525A-Biot was able to identify 24 triple-positive APS patients. ProTS525A-Biot may also be useful in future for the detection of other prothrombotic conditions apart from APS, such as COVID-19 (Chinnaraj et al., 2021).
Another diagnostic tool in the works is based on peptide IIa‐8.0‐biot‐2x, which is able to interact with antiphospholipid antibodies (aPLs) to a much larger extent (Moghbel et al., 2022).
Research on B Cells & T Cells in Autoimmune Diseases
If you live with an autoimmune disease, chances are that you’ve heard of T cells and B cells, and might even have underwent a biologic infusion to try and eliminate them, as they often trigger autoimmune disease activity when they go awry. T cells and B cells are lymphocytes, which are a type of white blood cell. As per the National Human Genome Research Institute, B cells produce antibodies that target invading bacteria, viruses and toxins, whilst T cells destroy the body’s own cells that have become infected, or have turned cancerous.
There has been some fascinating research into T cells and B cells, although they are still in the preclinical stages. According to Taylor et al. (2023):
“One such therapy is redirecting T cells to selectively kill anti-β2GPI antibody-producing B cells using chimeric autoantigen-TCRs (CATCRs), allowing T cells to bind autoantigen-specific B-cell receptors (BCRs) and induce selective cell death. Other B-cell therapies include monoclonal antibodies to B-cell activating factor (BAFF), which has been shown to exist at higher levels in many types of autoimmune diseases, including APS.”
In brief, T cells and B cells go hand in hand in the body, and targeting one will affect the other for better or for worse. Within the thymus gland, B cells play an active role in ‘training’ T cells on which cells to attack, and which to leave alone.
According to Afzali et al. (2024) in this latest research paper:
“CD40-induced B cell antigens, which, besides AQP4, comprise additional potentially disease-relevant autoantigens such as Anxa2, App and Cpd. Autoantibodies to ANXA2 and APP are associated with antiphospholipid syndrome and cerebral amyloid angiopathy-related inflammation.”
Thus, perhaps future treatments for Antiphospholipid Syndrome might target B cells and T cells more specifically, by ‘switching off’ the bad stuff whilst leaving the good alone.
Disruption of Neutrophilic Involvement in Antiphospholipid Syndrome
According to Papayannopoulos (2018), “Neutrophils are the most abundant innate immune effector cells of the human immune system”, and come with antimicrobials on a broad spectrum. Neutrophil extracellular traps (NETs) are a combination of chromatin fibres, DNA and histones, and they play an important role in immobilising and killing invasive microorganisms, thereby protecting against infection.
However, NETS are also sources of autoantigens and immunostimulatory proteins, and can stimulate autoimmune diseases such as Lupus (SLE), vasculitis and psoriasis (Kaplan and Radic, 2012). Apart from that, NETs play a role in blood coagulation both through platelet and coagulation cascade activation, and can contribute to the formation of both arterial and venous blood clots (Kmeťová et al., 2023).
Clinical trials in Primary Antiphospholipid Syndrome patients with no comorbidities have also shown that these individuals had high levels of anti-NET antibodies, which can potentially trigger the complement cascade (Zuo et al., 2023). Another study found that APS patients with a high risk of thrombosis had more NETs and activated protein C resistance (Foret et al., 2022).
With NETs being drivers of thromboinflammation in APS, research into disruption of this pathway is underway. Two FDA-approved medications with antineutrophilic properties are being studied, as well as selective modulation of immune cell activity. This could potentially result in neutralisation of adhesive properties of cells, thus reducing thrombosis in both arterial and venous vascular beds (Taylor et al., 2023).
Beta-2-Glycoprotein I (β2GPI) & Future Therapeutic Research in Relation to APS
There are only three proteins in the body that are able to up and down regulate the complement and coagulation systems, namely: C-reactive protein (CRP), thrombomodulin and Beta-2-Glycoprotein I (β2GPI). β2GPI is a unique five domain protein, and exists in open (J-shaped), S-twisted, and closed (O-shaped) conformations (McDonnell et al., 2020), and according to Ağar et al. (2010):
“In contrast to the circular conformation, the open fishhook-like conformation of β2GPI has a profound effect on the aPTT. Therefore, we propose that the conformation of β2GPI in plasma is predominantly circular.”
Coagulation from β2GPI is dependent on the surrounding environment, and can have an anticoagulant, antiplatelet and procoagulant effect (McDonnell et al., 2020). β2GPI is also known to bind to phospholipids and DNA, where interactions with DNA further extend to NETs (which we touched on previously) (Kmeťová et al., 2023).
According to McDonnell et al. (2020):
“These actions of β2GPI can be influenced by aβ2GPI antibodies present in patients with APS and may be potential therapeutic targets. Assays to measure levels of antibodies to β2GPI and to DI show promise in improving diagnosis and risk stratification of patients with APS. A number of proposed therapeutic agents that target β2GPI/aβ2GPI interactions are in development.”
In basic terms, what this means is that β2GPI has implications in coagulation and Antiphospholipid Syndrome. Future therapies may target the blocking of these interactions, in order to reduce the cascade of thrombosis.
One of these new potential therapeutics is A1-A1, which is a peptide that targets the fifth domain of β2GPI, in order to prevent binding to cell surfaces. Another potential therapeutic is TIFI, a cytomegalovirus capsid peptide, which also targets the fifth domain of β2GPI, and might be able to inhibit the thrombotic effects of IgG antibodies.
1N11 is a monoclonal antibody that has also been shown to target ß2GP1 to decrease binding of antiphospholipid antibodies (aPLs) to proteins. Recombinant domain I molecule is also able to bind to aPLs, which in turn prevents their adverse effects. Researchers are still trying to understand the full role of β2GPI as well as Antiphospholipid Syndrome. So whilst these therapeutics sound exciting, it will probably still be many years before we see them in practice (McDonnell et al., 2020; Fierro et al., 2022).
Aptamers / Next-Generation Thrombin Inhibitor Consisting of DNA Molecules
Finally, let’s take a look at aptamers. Aptamers are single-stranded oligonucleotides (DNA or RNA molecules) that are capable of binding to proteins or other cellular targets with great specificity (Arbuthnot, 2015; Ni et al., 2011). They are small in size, non-immunogenic, and there are different kinds that show promise in a variety of immune related treatments – from antibiotic alternatives, to autoimmune diseases such as Sjögren’s disease, to suppression of tumour growth. The good news is that many of these therapeutic aptamers are in the mid to late stage of clinical trials, and might be ready within 5 – 10 years (Yasmeen et al., 2020).
In relation to Antiphospholipid Syndrome and its potential complications, a new DNA drug to fight blood clots has been discovered. Heparin is used as an anticoagulation drug in APS patients during emergencies, but up to 3% of patients who are on heparin for various medical reasons develop heparin-induced thrombocytopenia (HIT), which causes the blood to clot instead. HIT is a life-threatening complication where platelet counts drop, and the patient is at risk of thrombosis. The medications used to treat HIT – argatroban and bivalirudin – have no antidotes.
Recently it has been found that the anti-thrombin DNA aptamer, M08s-1, might act as a promising antidote for HIT. It also does not cross over into the placenta of pregnant women. According to Nagano et al. (2023):
“The dimerized M08s-1-based aptamers had about 100-fold increased binding affinity to human and mouse thrombin compared with the monomer counterparts.”
Discovery of 2 Missing Enzymes in People with Antiphospholipid Syndrome
According to NaveenKumar et al. (2024):
“Many patients with antiphospholipid syndrome had decreased ectonucleotidase activity on neutrophils and platelets, which enabled extracellular nucleotides to trigger neutrophil-platelet aggregates. This phenotype was replicated by treating healthy neutrophils and platelets with patient-derived antiphospholipid antibodies or ectonucleotidase inhibitors.”
Goodwin from Michigan Medicine explains this unexpected research finding in simpler terms here. In brief, the enzymes CD39 and CD73 which normally work together to cool down inflammatory molecules known as adenosine triphosphate were not found in patients with APS. This results in instigation of cells like neutrophils and platelets that contribute to the blood coagulation cascade.
NaveenKumar et al. (2024) has also discovered that the receptors P2X7 and P2Y2, which play a role in platelets and neutrophils respectively, are key components in the inflammatory response from the accumulated adenosine triphosphate. Blocking these receptors on cells related to Antiphospholipid Syndrome returned them to a healthy state.
Research such as this matters so much even if there is little funding for research into Antiphospholipid Syndrome specifically. Every bit of knowledge helps medicine to advance as a whole.
Conclusion to the Latest Research on Antiphospholipid Syndrome
Currently the main form of APS management and treatment is with the use of blood thinning medications, which come their own sets of problems. So it’s interesting to see that the future direction of APS treatment delves quite a bit into more targeted therapies on a biological level.
I hope that this peek into the latest research on Antiphospholipid Syndrome has granted you some insight and hope into the possibilities of more effective APS diagnosis, prevention and treatments on the horizon. Perhaps some day, these fantastic researchers and doctors will find a cure as well. For now, take good care of yourself, and keep an eye out for the A – Z Antiphospholipid Syndrome guide to be released soon!
If you liked this article, sign up for our mailing list so you don’t miss out on our latest posts! You will also receive an e-book full of uplifting messages, quotes and illustrations, as a token of appreciation.
-
Read Related Posts:
- Chronic Illness Quotes to Inspire, Motivate, Grieve, Hope & Laugh About
- 12 Visible Evidence of a Body Gone Rogue (Is Invisible Illness Truly Invisible?)
- Fun & Productive Things to Do on Digital Devices After Knee Surgery
- Useful Things to Do While on Bed Rest After Surgery: Education, Advocacy & Volunteering
- Loss of Identity with Chronic Illness & The Plot Twist: Sharpened Self-Worth
Pin to Your Latest Research on Antiphospholipid Syndrome Boards:


- Afzali, A. M., Nirschl, L., Sie, C., Pfaller, M., Ulianov, O., Hassler, T., … & Korn, T. (2024). B cells orchestrate tolerance to the neuromyelitis optica autoantigen AQP4. Nature, 627, 407-415. https://doi.org/10.1038/s41586-024-07079-8
- Ağar, Ç., van Os, G. M., Mörgelin, M., Sprenger, R. R., Marquart, J. A., Urbanus, R. T., … & de Groot, P. G. (2010). β2-Glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood, The Journal of the American Society of Hematology, 116(8), 1336-1343. https://doi.org/10.1182/blood-2009-12-260976
- Arbuthnot, P. (2015). Chapter 5 – Delivery of Antiviral Nucleic Acids with Nonviral Vectors. Gene Therapy for Viral Infections. 127-150. https://doi.org/10.1016/B978-0-12-410518-8.00005-3
- Chinnaraj, M., Pengo, V., & Pozzi, N. (2021). A novel ELISA assay for the detection of anti-prothrombin antibodies in antiphospholipid syndrome patients at high risk of thrombosis. Frontiers in Immunology, 12, 741589. https://doi.org/10.3389/fimmu.2021.741589
- Cleveland Clinic. (2022). Heparin-Induced Thrombocytopenia. Cleveland Clinic. Retrieved from: https://my.clevelandclinic.org/health/diseases/24014-heparin-induced-thrombocytopenia
- Fierro, J. J., Velásquez, M., Cadavid, A. P., & de Leeuw, K. (2022). Effects of anti‐beta 2‐glycoprotein 1 antibodies and its association with pregnancy‐related morbidity in antiphospholipid syndrome. American Journal of Reproductive Immunology, 87(1), e13509. https://doi.org/10.1111/aji.13509
- Foret, T., Dufrost, V., Salomon du Mont, L., Costa, P., Lakomy, C., Lagrange, J., … & Wahl, D. (2022). A new pro-thrombotic mechanism of neutrophil extracellular traps in antiphospholipid syndrome: impact on activated protein C resistance. Rheumatology, 61(7), 2993-2998. https://doi.org/10.1093/rheumatology/keab853
- Goodwin, V. (April 18, 2024). Helpful enzymes vanish in many patients with antiphospholipid syndrome. Michigan Medicine. Retrieved from: https://www.michiganmedicine.org/health-lab/helpful-enzymes-vanish-many-patients-antiphospholipid-syndrome
- Hospital for Special Surgery. (2023). New Antiphospholipid Syndrome Research Findings Presented at ACR Convergence 2023. Hospital for Special Surgery. Retrieved from: https://news.hss.edu/new-antiphospholipid-syndrome-research-findings-presented-at-acr-convergence-2023/
- Kaplan, M. J., & Radic, M. (2012). Neutrophil extracellular traps: double-edged swords of innate immunity. The Journal of Immunology, 189(6), 2689-2695. https://doi.org/10.4049/jimmunol.1201719
- Kmeťová, K., Lonina, E., Yalavarthi, S., Levine, J. S., Hoy, C. K., Sarosh, C., … & Knight, J. S. (2023). Interaction of the antiphospholipid syndrome autoantigen beta-2 glycoprotein I with DNA and neutrophil extracellular traps. Clinical Immunology, 255, 109714. https://doi.org/10.1016/j.clim.2023.109714
- McDonnell, T., Wincup, C., Buchholz, I., Pericleous, C., Giles, I., Ripoll, V., … & Rahman, A. (2020). The role of beta-2-glycoprotein I in health and disease associating structure with function: More than just APS. Blood reviews, 39, 100610. https://doi.org/10.1016/j.blre.2019.100610
- Moghbel, M., Roth, A., Baptista, D., Miteva, K., Burger, F., Montecucco, F., … & Brandt, K. J. (2022). Epitope of antiphospholipid antibodies retrieved from peptide microarray based on R39‐R43 of β2‐glycoprotein I. Research and Practice in Thrombosis and Haemostasis, 6(7), e12828. https://doi.org/10.1002/rth2.12828
- Nagano, M., Kubota, K., Sakata, A., Nakamura, R., Yoshitomi, T., Wakui, K., & Yoshimoto, K. (2023). A neutralizable dimeric anti-thrombin aptamer with potent anticoagulant activity in mice. Molecular Therapy-Nucleic Acids, 33, 762-772. https://doi.org/10.1016/j.omtn.2023.07.038
- National Human Genome Research Institute. (2024). Lymphocyte. National Human Genome Research Institute. Retrieved from: https://www.genome.gov/genetics-glossary/Lymphocyte
- NaveenKumar, S. K., Tambralli, A., Fonseca, B. M., Yalavarthi, S., Liang, W., Hoy, C. K., Sarosh, C., Rysenga, C. E., Ranger, C. H., Vance, C. E., Madison, J. A., Orsi, F. A., Sood, S. L., Schaefer, J. K., Zuo, Y., & Knight, J. S. (2024). Low ectonucleotidase activity and increased neutrophil-platelet aggregates in patients with antiphospholipid syndrome. Blood, 143(12), 1193–1197. https://doi.org/10.1182/blood.2023022097
- Ni, X., Castanares, M., Mukherjee, A., & Lupold, S. E. (2011). Nucleic acid aptamers: clinical applications and promising new horizons. Current medicinal chemistry, 18(27), 4206-4214. https://doi.org/10.2174/092986711797189600
- Papayannopoulos, V. (2018). Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology, 18(2), 134-147. https://doi.org/10.1038/nri.2017.105
- Taylor, A., Kumar, S., & Pozzi, N. (2023). Forecasting the Future of Antiphospholipid Syndrome: Prospects and Challenges. Missouri Medicine, 120(5), 359-366. Retrieved from: https://www.ncbi.nlm.nih.gov/pubmed/37841574
- Technical University of Munich (TUM). (2024). Possible trigger for autoimmune diseases discovered : B cells teach T cells which targets must not be attacked. ScienceDaily. Retrieved from: https://www.sciencedaily.com/releases/2024/02/240221160321.htm
- University of Tokyo. (2023). A new DNA drug to fight blood clots. ScienceDaily. Retrieved from: https://www.sciencedaily.com/releases/2023/08/230821114401.htm
- Yasmeen, F., Seo, H., Javaid, N., Kim, M. S., & Choi, S. (2020). Therapeutic interventions into innate immune diseases by means of aptamers. Pharmaceutics, 12(10), 955. https://doi.org/10.3390/pharmaceutics12100955
- Zuo, Y., Navaz, S., Tsodikov, A., Kmetova, K., Kluge, L., Ambati, A., … & Branch, D. W. (2023). Anti–Neutrophil Extracellular Trap Antibodies in Antiphospholipid Antibody–Positive Patients: Results From the Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking Clinical Database and Repository. Arthritis & Rheumatology, 75(8), 1407-1414. https://doi.org/10.1002/art.42489
References:
Taste the Rainbow: Vibrant Gluten-Free Salsa Recipes for Every Palate
Who doesn’t love salsa? The savory blend of tomatoes, onions, chili peppers, cilantro, and lime juice combined to create an irresistible sauce. Or better yet, how about the summer sweetness of juicy mangoes, flavorful pineapples, and strawberries bursting with flavor. With an array of fresh […]
mind-&-emotionsWho doesn’t love salsa? The savory blend of tomatoes, onions, chili peppers, cilantro, and lime juice combined to create an irresistible sauce. Or better yet, how about the summer sweetness of juicy mangoes, flavorful pineapples, and strawberries bursting with flavor. With an array of fresh flavors, these gluten-free salsa recipes are your ticket to a flavor-packed fiesta!

While salsa may have started with Mexican roots, it wasn’t long before its flavor extended well across the globe. Nowadays every culture has its own customized version. Whether accompanying a big bowl of tortilla chips, spread over an omelet, or served alongside your favorite grilled barbecue, salsa adds life to any dish.
If spicy salsa is your thing, fresh jalapeno, chipotle peppers or even chili peppers can give your salsa an extra kick.
What type of tomatoes make the best salsa
As you’ll see from these vibrant salsa recipes below, salsa doesn’t always require tomatoes. But if you’re going to opt for a version with tomatoes, Roma tomatoes are the best to use for salsa. This is a meaty, tangy variety with fewer seeds.
While canned tomatoes can be used, fresh tomatoes are best.
29 Gluten-Free Salsa Recipes
While every effort has been made to categorize these recipes, always use caution and your own judgment when following someone else’s recipe.
For sensitive, gluten-free individuals, I recommend looking for the “gluten-free” label on canned ingredients and seasonings. For more information, check out 10 Gluten Free Life Hacks.











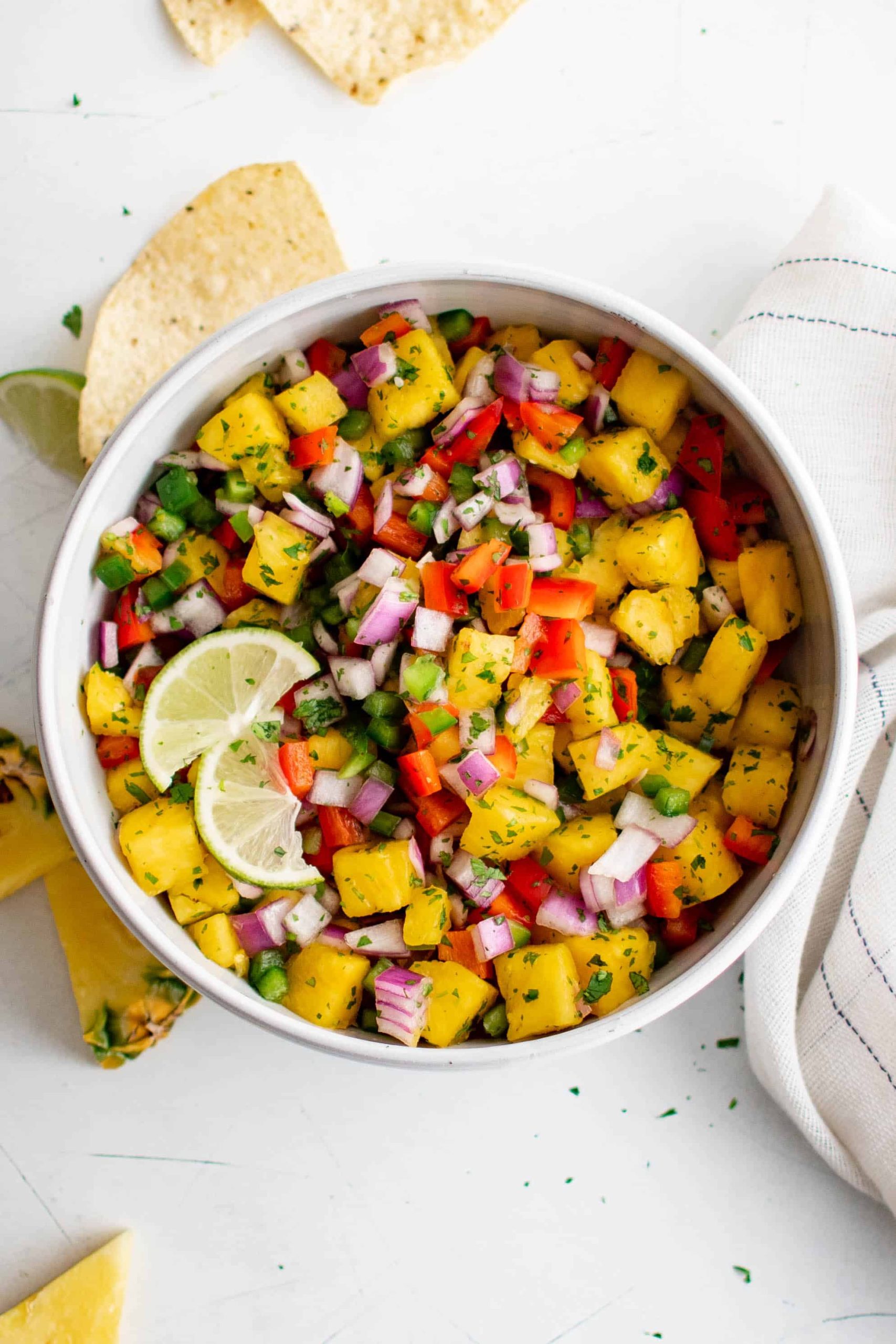





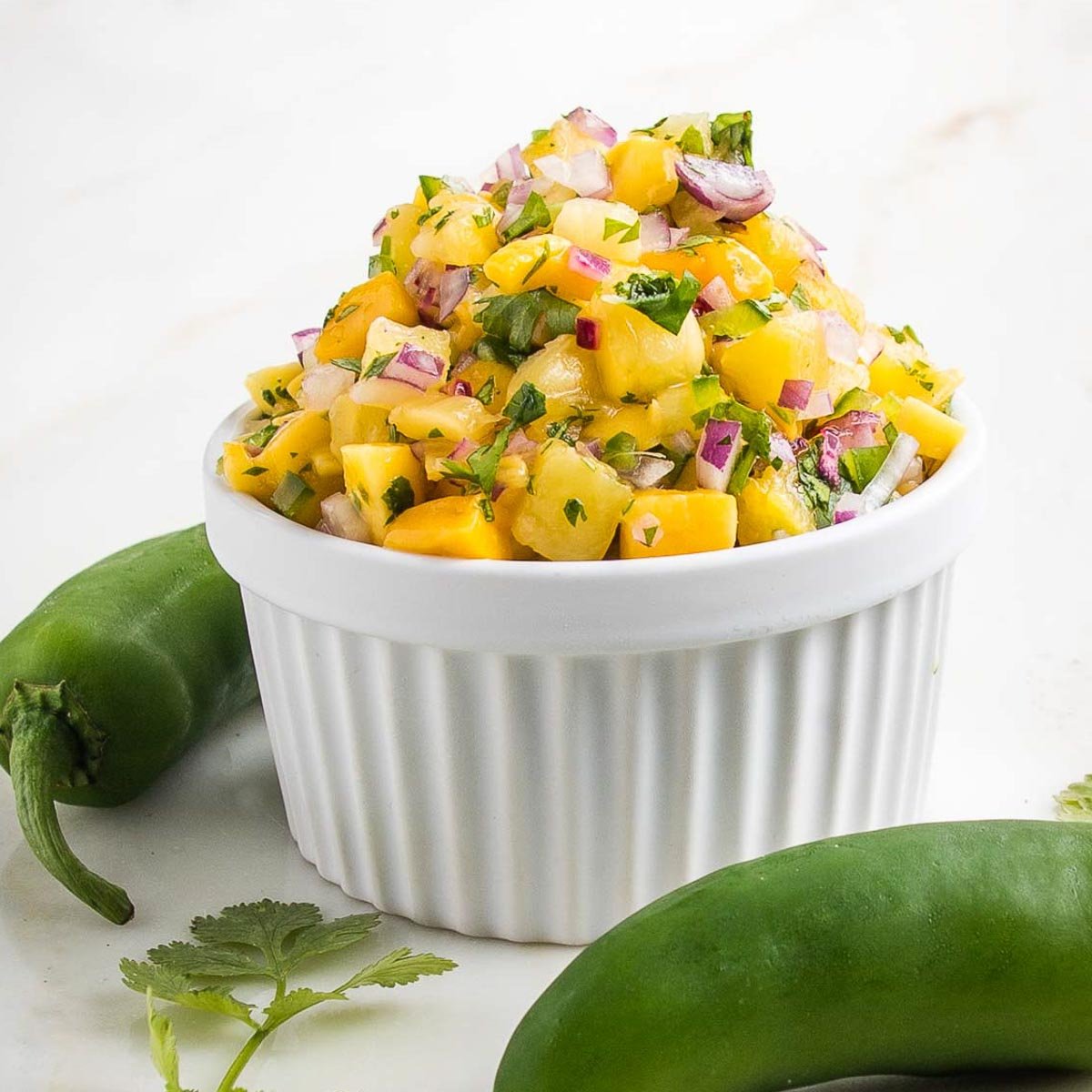


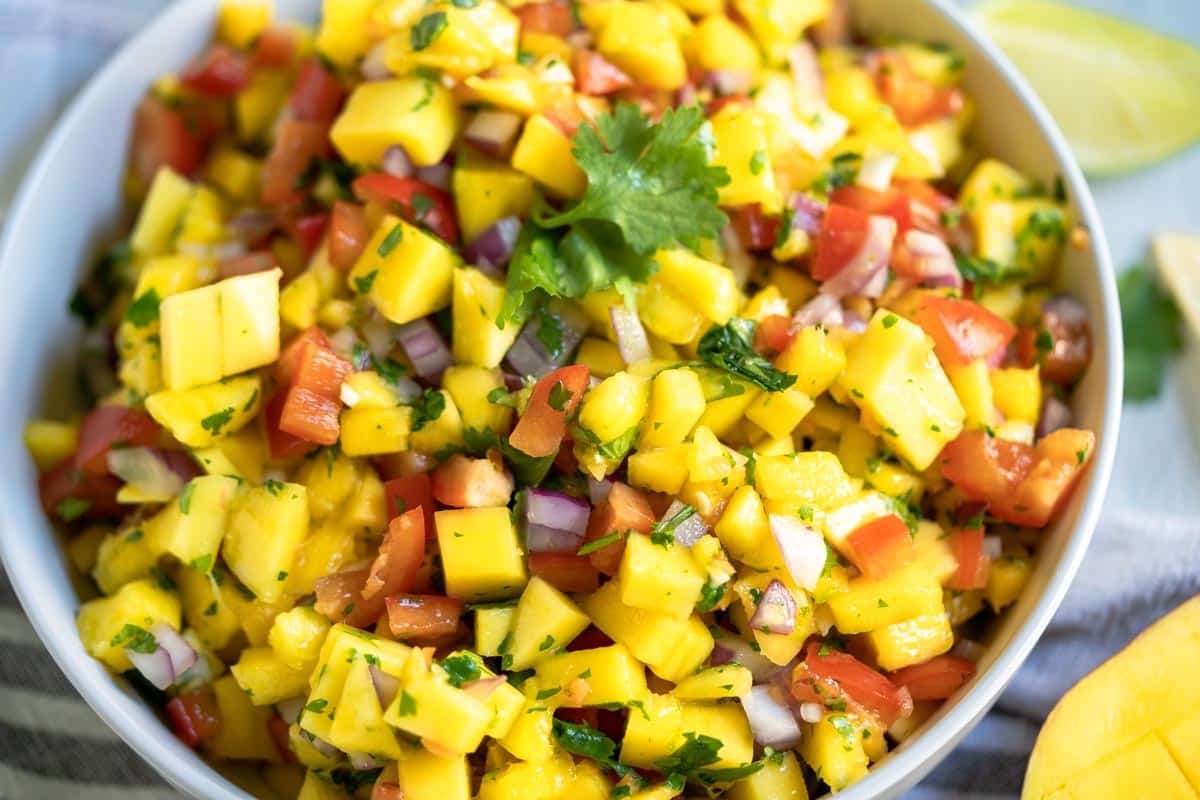








You May Also Want To Read
56 Sizzling Summer Grill Recipes That Will Make Your Mouth Water
30+ Delicious Dairy and Gluten-Free BBQ Side Dishes to Try This Summer
These gluten-free salsa recipes offer exciting flavors with diverse ingredients. Whether you’re celebrating Cinco de Mayo, Taco Tuesday, or simply looking to spice up your grilled meats, these recipes prove that you don’t have to sacrifice taste just because you’re gluten-free. With a variety of options to suit every palate and occasion, you can enjoy the vibrant flavors with joy.
The post Taste the Rainbow: Vibrant Gluten-Free Salsa Recipes for Every Palate appeared first on Health Uprising Now.














